Fluidics For Preclinical Market Report
Published Date: 31 January 2026 | Report Code: fluidics-for-preclinical
Fluidics For Preclinical Market Size, Share, Industry Trends and Forecast to 2033
This report presents an in-depth analysis of the Fluidics For Preclinical market, providing insights into market size, trends, and growth forecasts from 2023 to 2033. A comprehensive overview of market dynamics, key players, and regional performances is included to facilitate strategic planning and decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
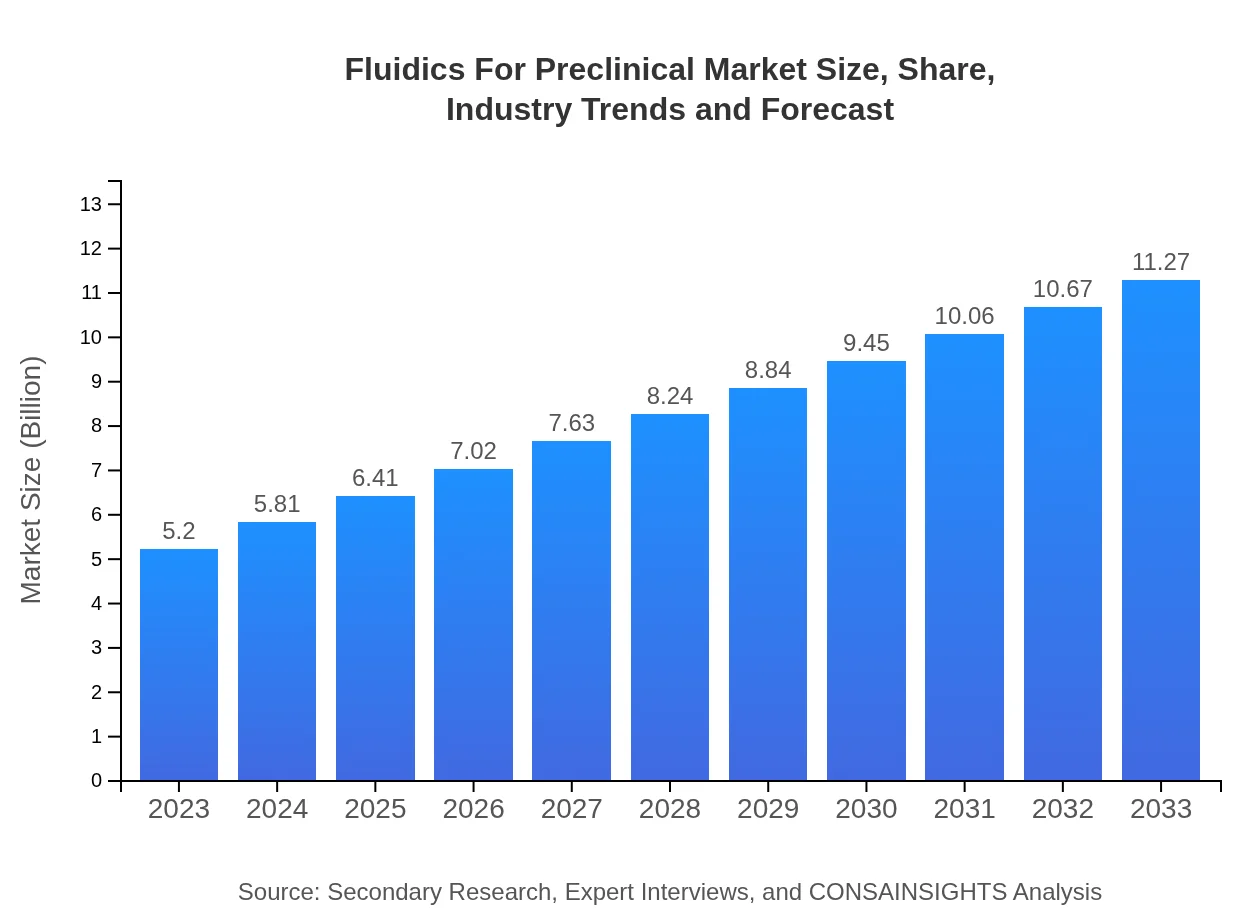
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $11.27 Billion |
| Top Companies | Merck Group, Thermo Fisher Scientific, Agilent Technologies, Abbott Laboratories, Danaher Corporation |
| Last Modified Date | 31 January 2026 |
Fluidics For Preclinical Market Overview
Customize Fluidics For Preclinical Market Report market research report
- ✔ Get in-depth analysis of Fluidics For Preclinical market size, growth, and forecasts.
- ✔ Understand Fluidics For Preclinical's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Fluidics For Preclinical
What is the Market Size & CAGR of Fluidics For Preclinical market in 2023?
Fluidics For Preclinical Industry Analysis
Fluidics For Preclinical Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Fluidics For Preclinical Market Analysis Report by Region
Europe Fluidics For Preclinical Market Report:
The European market, valued at $1.48 billion in 2023, is projected to reach $3.21 billion by 2033, growing at a CAGR of 8.06%. Strong regulatory frameworks and collaboration between academia and industry are key drivers for market expansion.Asia Pacific Fluidics For Preclinical Market Report:
In the Asia-Pacific region, the Fluidics For Preclinical market is expected to grow from $1.10 billion in 2023 to $2.38 billion by 2033, reflecting a CAGR of 8.02%. The region benefits from rising investments in biotech and pharma sectors as well as increasing outsourcing of research activities.North America Fluidics For Preclinical Market Report:
North America is anticipated to dominate the market, growing from $1.82 billion in 2023 to $3.94 billion in 2033, with a CAGR of 8.08%. This region is marked by strong investments in R&D, established pharmaceutical companies, and a focus on innovative preclinical technologies.South America Fluidics For Preclinical Market Report:
The South American market is projected to grow from $0.52 billion in 2023 to $1.13 billion in 2033, at a CAGR of 8.24%. Factors contributing to growth include improvements in healthcare infrastructure and increasing demand for drug development.Middle East & Africa Fluidics For Preclinical Market Report:
The Middle East and Africa region represents a smaller, yet growing market, expected to increase from $0.29 billion in 2023 to $0.62 billion by 2033, at a CAGR of 8.14%. Growing research activities and investment in healthcare will bolster market prospects.Tell us your focus area and get a customized research report.
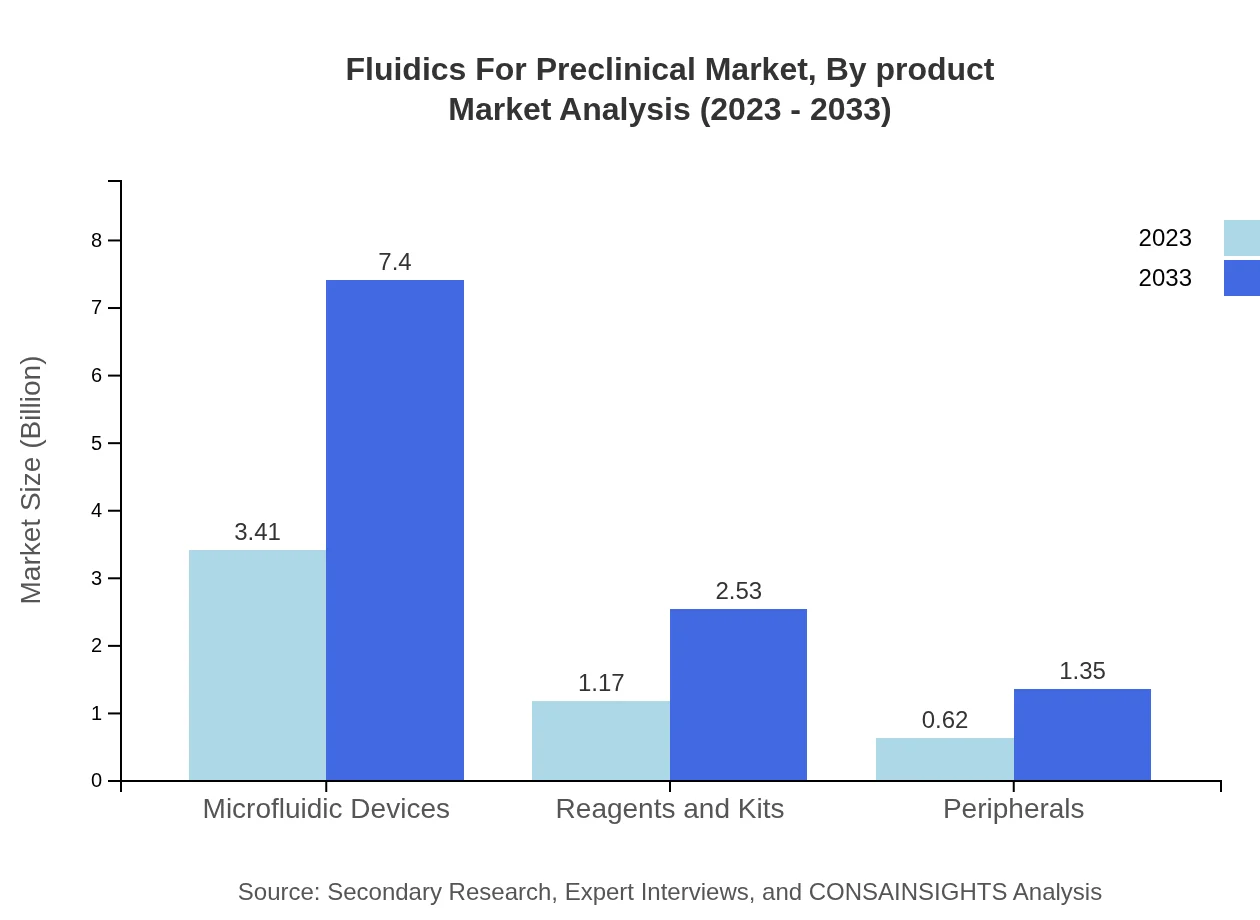
Fluidics For Preclinical Market Analysis By Product
Microfluidic Devices dominate the Fluidics For Preclinical market, projected to grow from $3.41 billion in 2023 to $7.40 billion in 2033, capturing 65.63% market share. Reagents and Kits follow with a significant presence, expected to rise from $1.17 billion to $2.53 billion. Peripherals, while smaller, are also essential, expanding from $0.62 billion to $1.35 billion, contributing to a versatile product ecosystem.
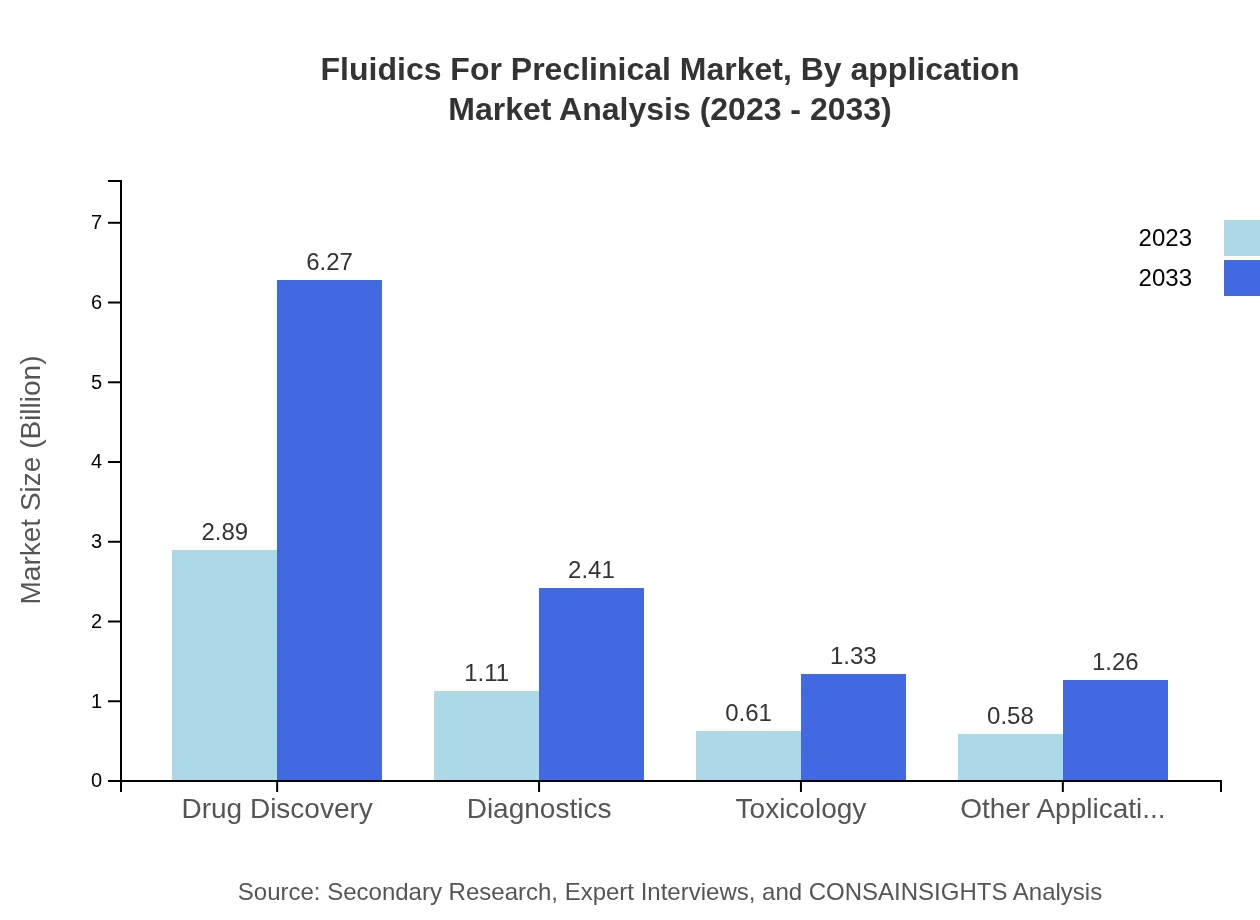
Fluidics For Preclinical Market Analysis By Application
The Drug Discovery segment retains the largest market share of 55.59%, projected to grow from $2.89 billion in 2023 to $6.27 billion. Diagnostics applications follow with a market size increase from $1.11 billion to $2.41 billion. Toxicology and other applications also indicate steady growth, reflecting broad utility in preclinical settings.
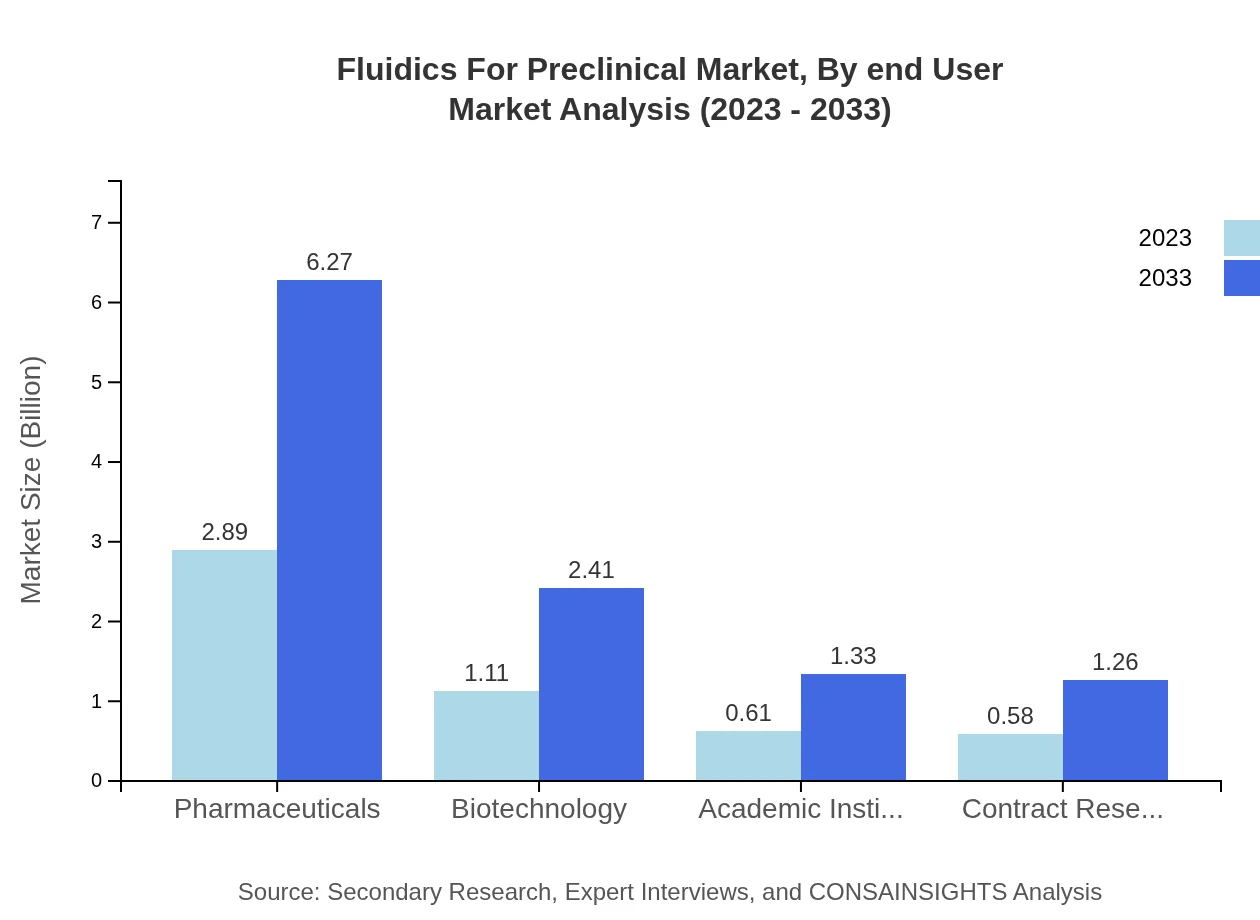
Fluidics For Preclinical Market Analysis By End User
Pharmaceutical companies account for a significant market share, particularly with a size projection from $2.89 billion to $6.27 billion. Biotechnology firms and academic institutions also play pivotal roles, contributing to innovations in fluidic technologies, alongside the growing involvement of Contract Research Organizations (CROs).
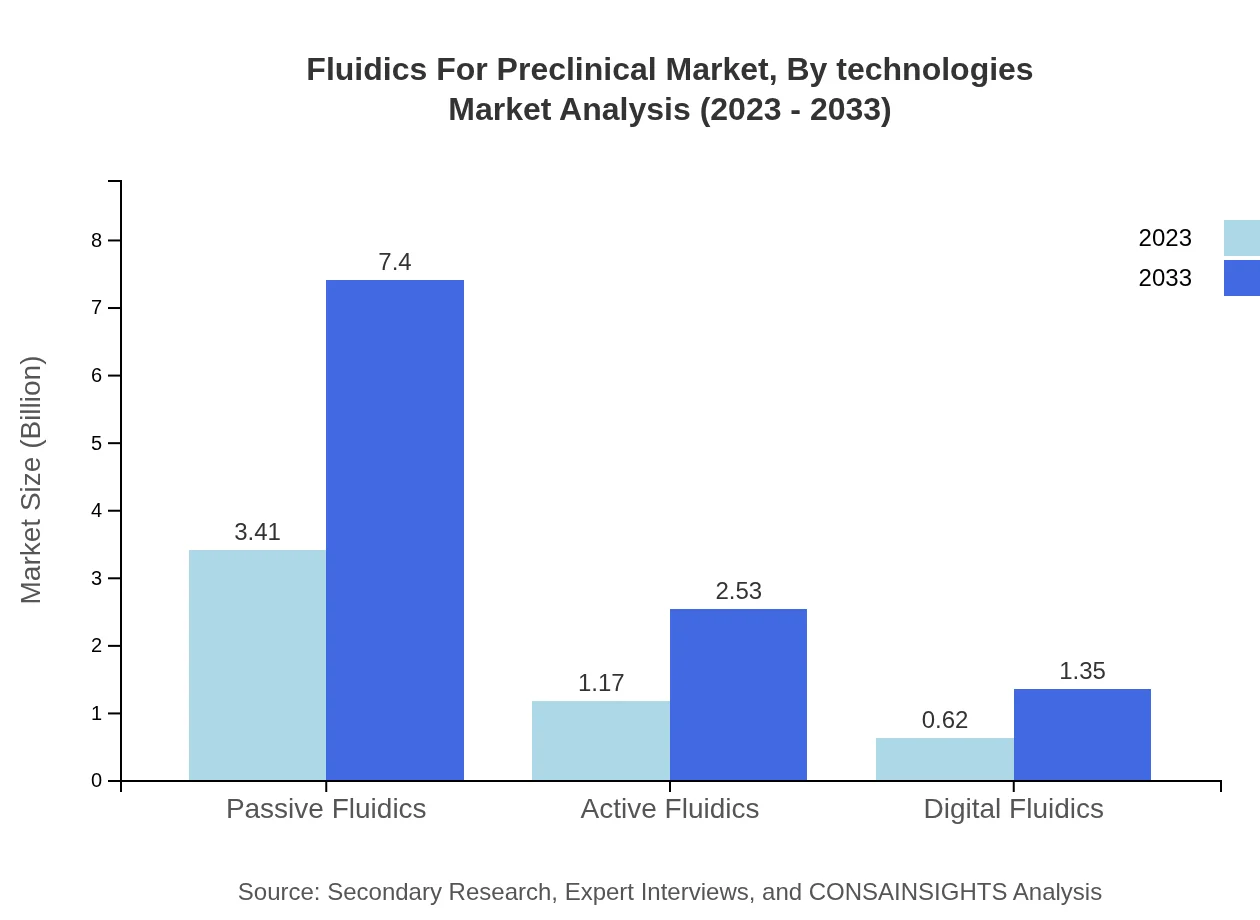
Fluidics For Preclinical Market Analysis By Technologies
The market for Passive Fluidics leads, showing growth from $3.41 billion to $7.40 billion, while Active Fluidics and Digital Fluidics exhibit promising advancements. The preference for automated and digitalized fluidic solutions continues to rise, reflecting changing laboratory needs and technological advances.
Fluidics For Preclinical Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Fluidics For Preclinical Industry
Merck Group:
Merck Group is a global leader in science and technology, with a strong portfolio in microfluidics and laboratory automation solutions.Thermo Fisher Scientific:
Thermo Fisher Scientific offers an extensive range of fluidics products, widely utilized in biopharma and academic research for preclinical solutions.Agilent Technologies:
Agilent Technologies specializes in providing innovative solutions in life sciences, including state-of-the-art fluidic devices for labs.Abbott Laboratories:
Abbott Laboratories is prominent in the diagnostics sector, integrating fluidics technology into their innovative preclinical and clinical solutions.Danaher Corporation:
Danaher Corporation operates in diagnostics and life sciences, providing advanced fluidics technologies to support preclinical research.We're grateful to work with incredible clients.









FAQs
What is the market size of Fluidics for Preclinical?
The Fluidics for Preclinical market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 7.8% driving its growth through to 2033, where it is expected to expand significantly.
What are the key market players or companies in the Fluidics for Preclinical industry?
Key players in the Fluidics for Preclinical industry include major biotechnology and pharmaceutical companies, equipment manufacturers specializing in microfluidics, and research organizations focused on drug discovery and diagnostics.
What are the primary factors driving the growth in the Fluidics for Preclinical industry?
Growth drivers include technological advancements in microfluidics, increasing investments in drug development, the rising demand for personalized medicine, and the need for more efficient diagnostic tools in preclinical studies.
Which region is the fastest Growing in the Fluidics for Preclinical?
The Asia Pacific region is the fastest-growing market in Fluidics for Preclinical, with market size expected to rise from $1.10 billion in 2023 to $2.38 billion by 2033, driven by expanding biotech sectors.
Does ConsaInsights provide customized market report data for the Fluidics for Preclinical industry?
Yes, ConsaInsights offers customized market report data for the Fluidics for Preclinical industry, allowing clients to obtain tailored insights and analyses specific to their particular business needs and interests.
What deliverables can I expect from this Fluidics for Preclinical market research project?
Deliverables from the Fluidics for Preclinical market research project include detailed market analysis, insights into trends, competitive landscape reports, regional breakdowns, and tailored recommendations for strategic planning.
What are the market trends of Fluidics for Preclinical?
Current market trends include a surge in adoption of microfluidic devices, integration of AI in diagnostics, growing collaborations between companies, and the shift towards automation in laboratory settings to enhance research workflows.