Gastrointestinal Gi Stent Market Report
Published Date: 31 January 2026 | Report Code: gastrointestinal-gi-stent
Gastrointestinal Gi Stent Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Gastrointestinal Gi Stent market, including market trends, size, and forecasts from 2023 to 2033. It covers industry insights, segmentation, regional performance, and key players, offering valuable information for stakeholders and decision-makers.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
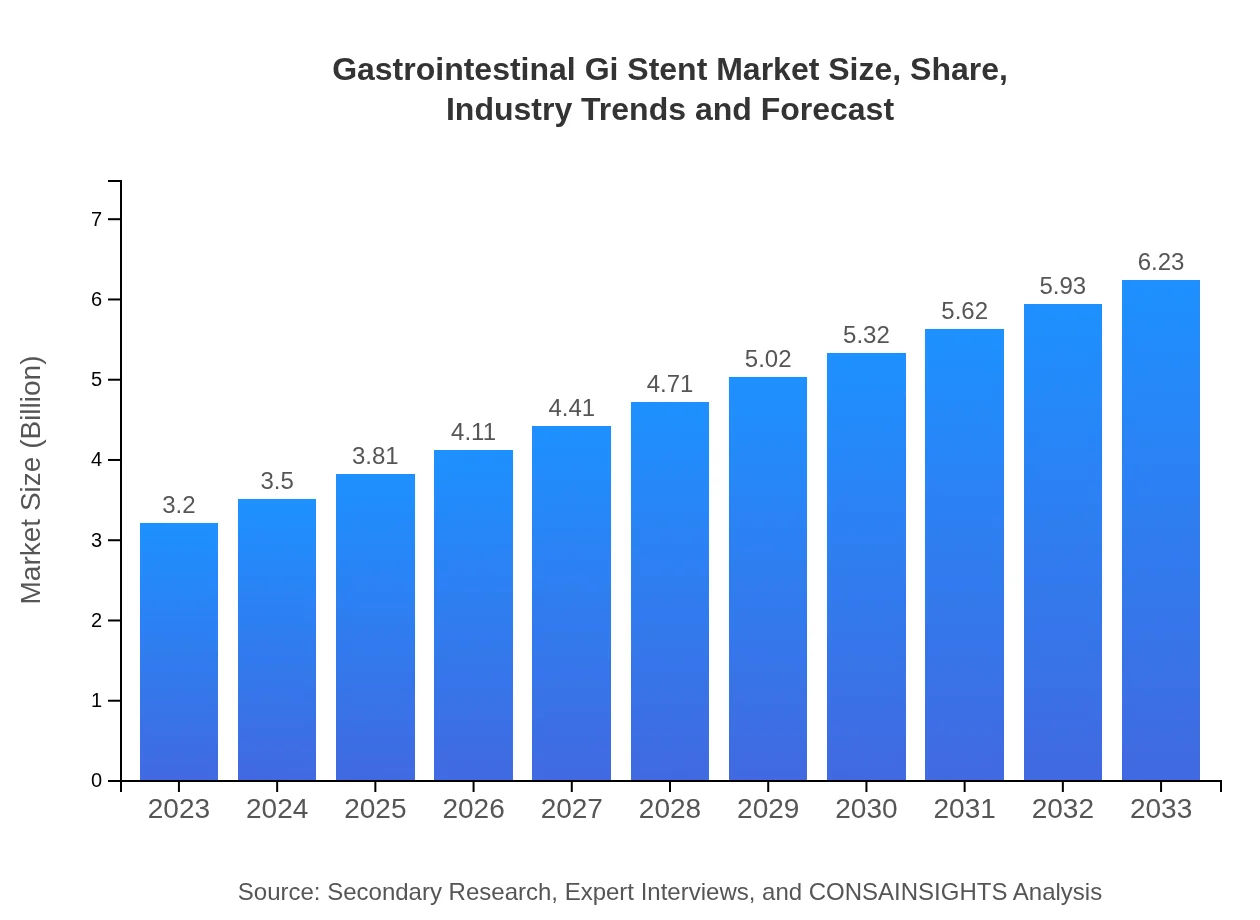
| 2023 Market Size | $3.20 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $6.23 Billion |
| Top Companies | Medtronic , Boston Scientific, Cook Medical, Johnson & Johnson, Abbott Laboratories |
| Last Modified Date | 31 January 2026 |
Gastrointestinal Gi Stent Market Overview
Customize Gastrointestinal Gi Stent Market Report market research report
- ✔ Get in-depth analysis of Gastrointestinal Gi Stent market size, growth, and forecasts.
- ✔ Understand Gastrointestinal Gi Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Gastrointestinal Gi Stent
What is the Market Size & CAGR of Gastrointestinal Gi Stent market in 2023?
Gastrointestinal Gi Stent Industry Analysis
Gastrointestinal Gi Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Gastrointestinal Gi Stent Market Analysis Report by Region
Europe Gastrointestinal Gi Stent Market Report:
Europe is experiencing growth due to increasing awareness regarding gastrointestinal health and significant advancements in stenting technologies. The market size is expected to grow from $0.80 billion in 2023 to $1.55 billion by 2033.Asia Pacific Gastrointestinal Gi Stent Market Report:
The Asia Pacific region is anticipated to grow significantly, with the market size projected to reach $1.23 billion by 2033, up from $0.63 billion in 2023. This growth is driven by an increasing patient population, improving healthcare infrastructure, and a rising trend towards advanced surgical techniques.North America Gastrointestinal Gi Stent Market Report:
North America remains the largest market, projected to grow from $1.18 billion in 2023 to $2.30 billion by 2033. Factors driving this growth include advanced healthcare systems, a high prevalence of gastrointestinal diseases, and strong R&D investments from key players.South America Gastrointestinal Gi Stent Market Report:
The South American market is expected to grow steadily, with projections of reaching $0.51 billion by 2033, an increase from $0.26 billion in 2023. The expansion of healthcare access and increasing investments in health technologies are expected to propel market growth.Middle East & Africa Gastrointestinal Gi Stent Market Report:
The market in the Middle East and Africa is projected to grow from $0.33 billion in 2023 to $0.64 billion by 2033, largely driven by increasing healthcare investments and enhancements in patient care facilities.Tell us your focus area and get a customized research report.
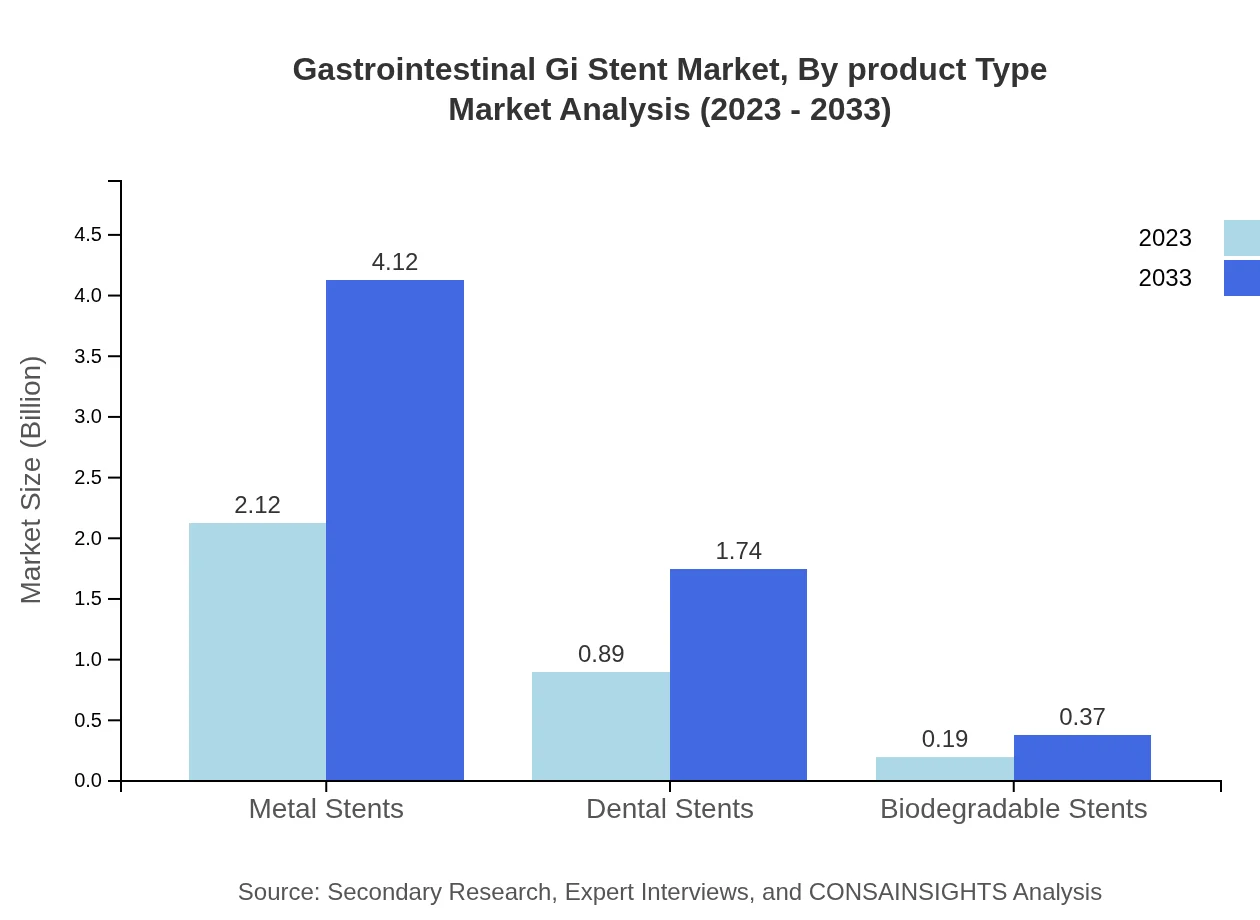
Gastrointestinal Gi Stent Market Analysis By Product Type
The analysis of the gastrointestinal stent market by product type indicates that Metal Stents lead the market with a share of 66.13% in both 2023 and 2033. Polymeric Stents are gaining traction with a consistent share of 27.96%, while Composite Stents maintain a minority share of 5.91%. The preference for Metal Stents is attributed to their strength and durability, while the increasing acceptance of Polymeric Stents stems from their biodegradability.
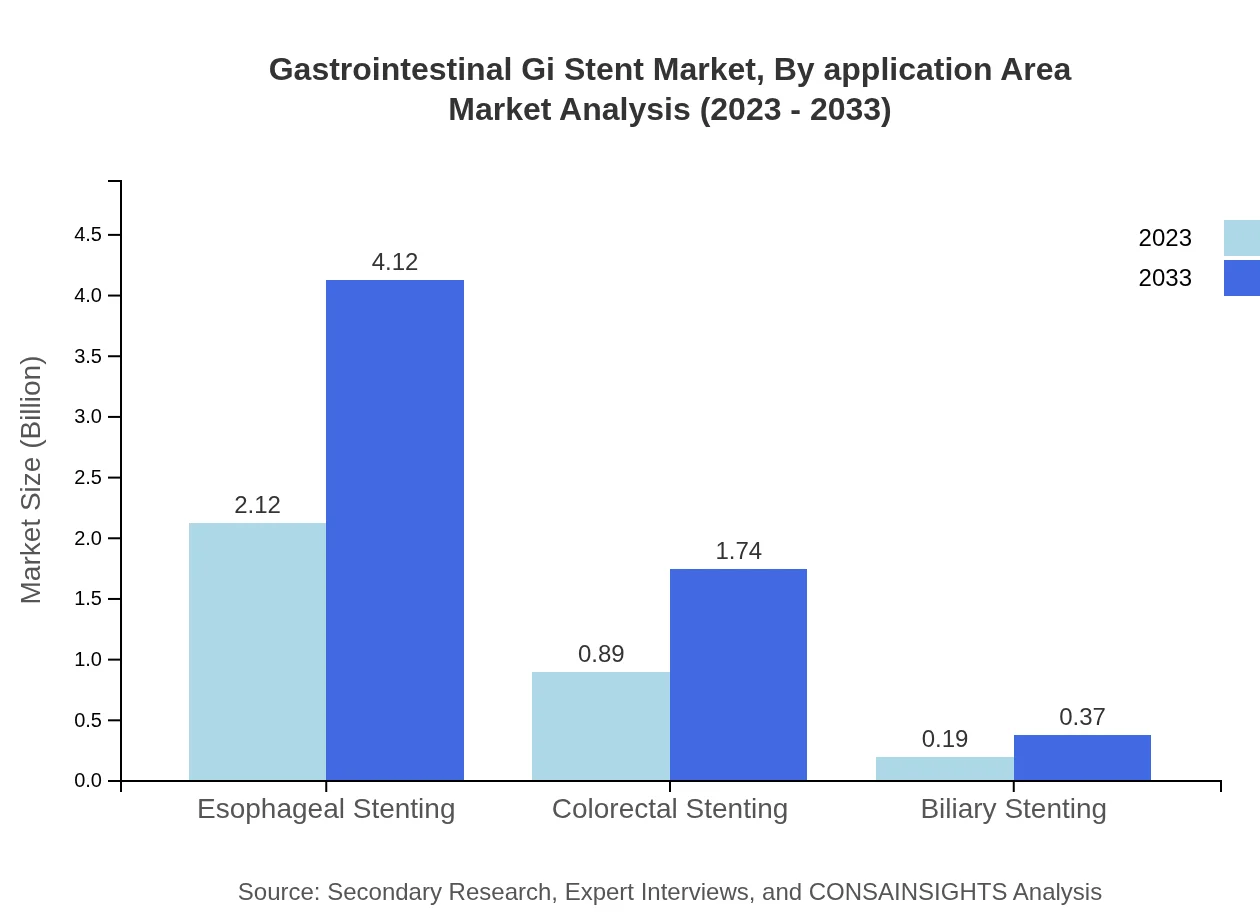
Gastrointestinal Gi Stent Market Analysis By Application Area
In the application area segment, Esophageal Stenting accounts for the largest market share of 66.13% in both 2023 and 2033. Colorectal Stenting follows with 27.96%, while Biliary Stenting constitutes 5.91%. The high prevalence of esophageal diseases is the major factor contributing to this dominant share.
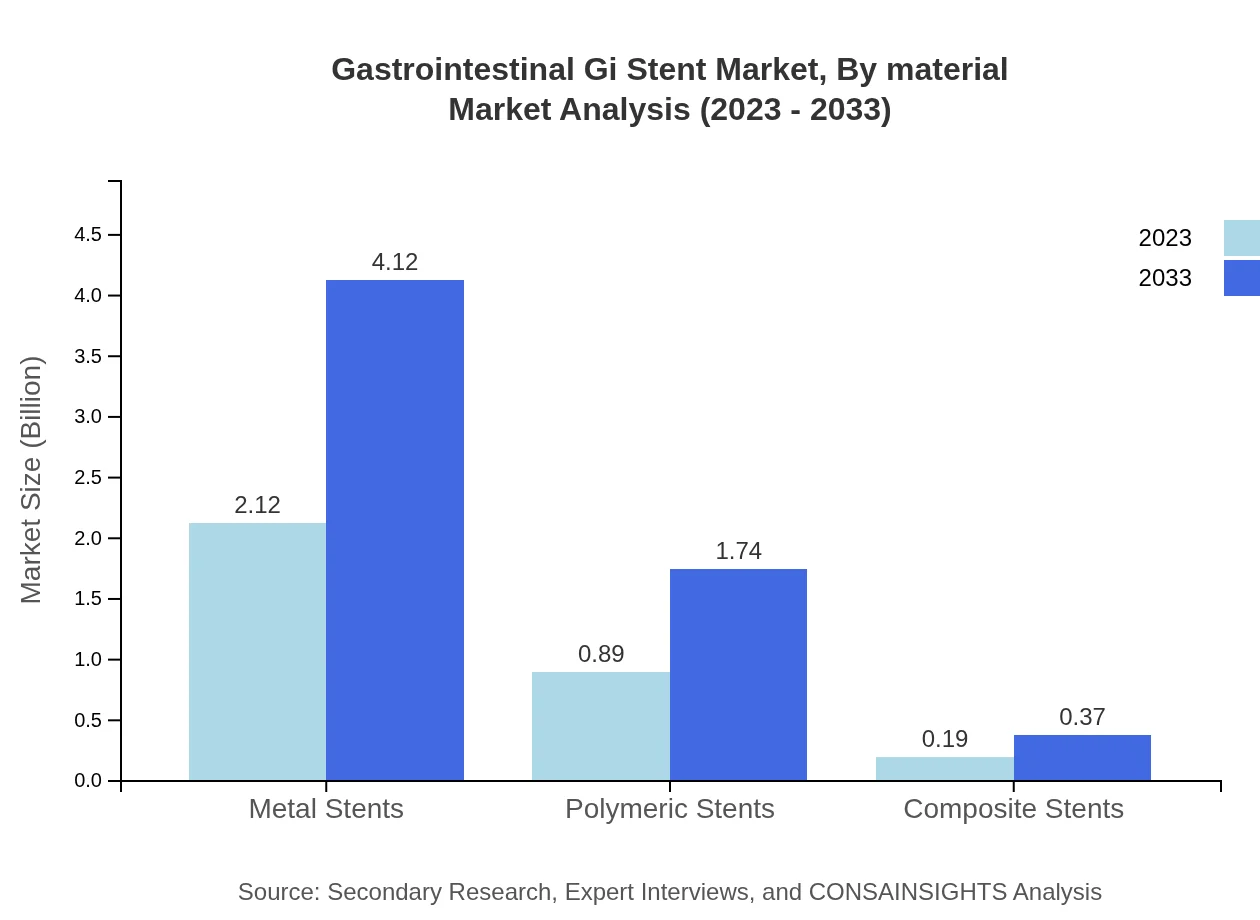
Gastrointestinal Gi Stent Market Analysis By Material
The market by material shows that Non-biodegradable stents are leading with 66.13% in 2023 and remaining unchanged in 2033. Biodegradable stents, while smaller in market share at 5.91%, are anticipated to see growth as patient acceptance increases.
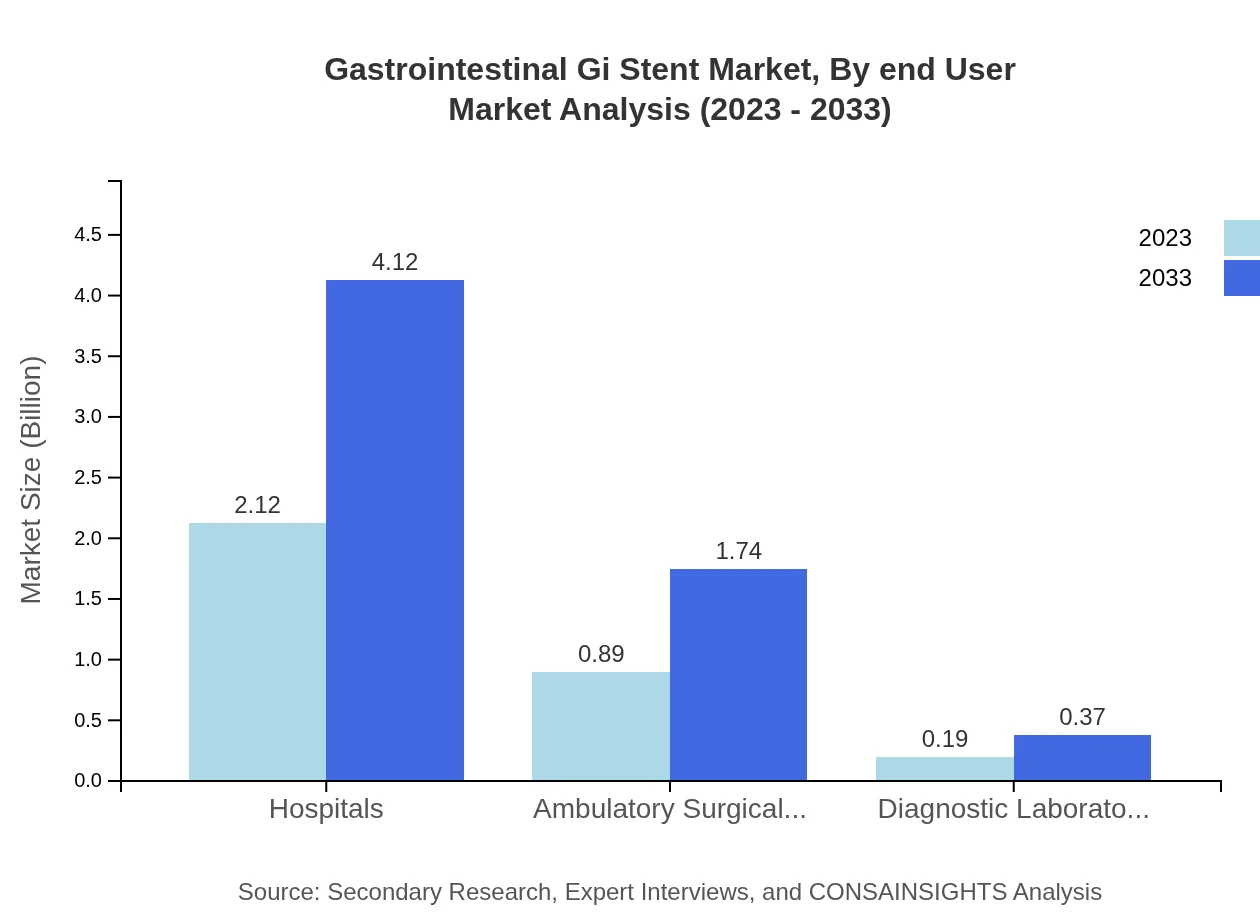
Gastrointestinal Gi Stent Market Analysis By End User
Hospitals dominate the market with a significant share of 66.13% in both 2023 and 2033, driven by higher patient admissions and advanced treatment capabilities. Ambulatory Surgical Centers contribute 27.96% to the market, while Diagnostic Laboratories account for 5.91%.
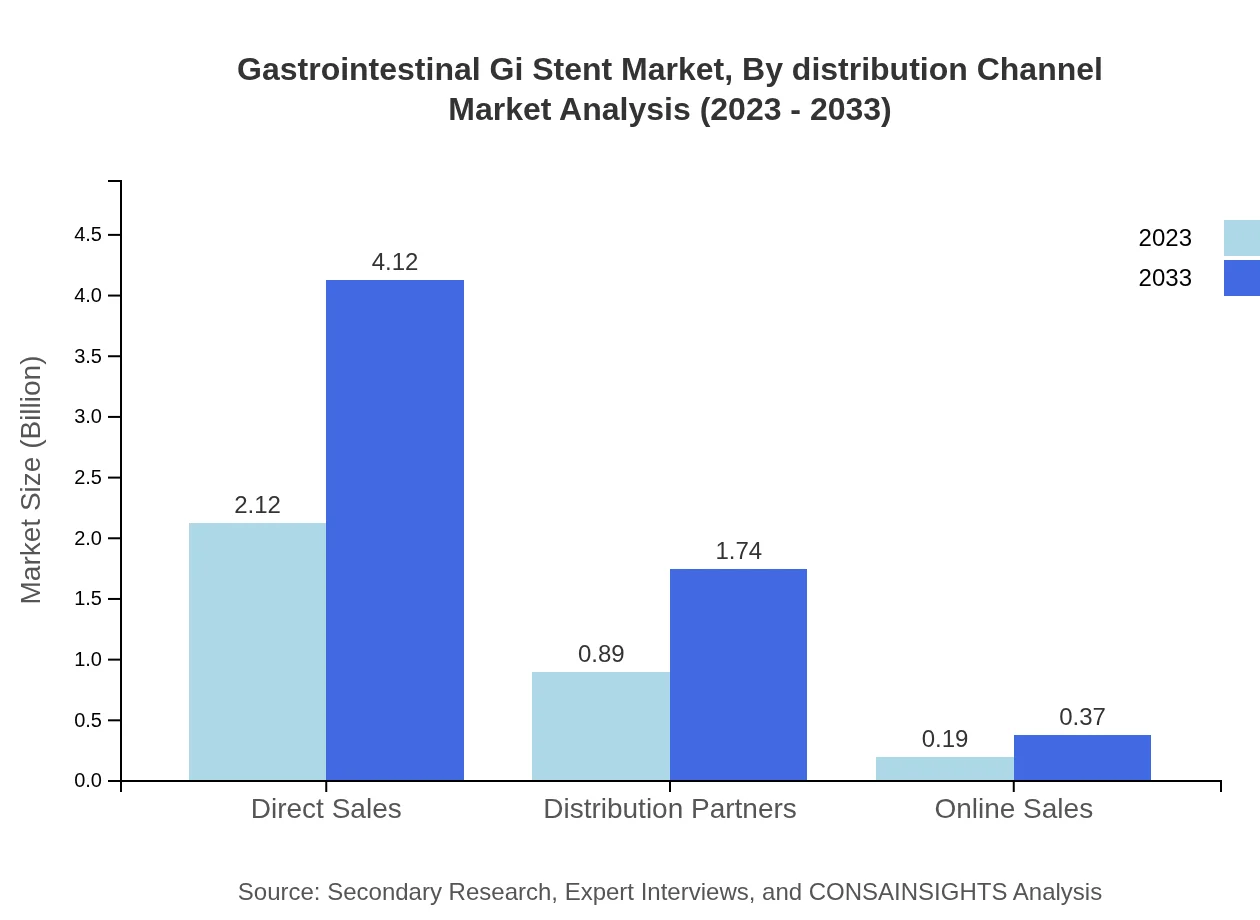
Gastrointestinal Gi Stent Market Analysis By Distribution Channel
Direct Sales channels dominate with a share of 66.13%, followed by Distribution Partners at 27.96% and Online Sales at 5.91%. The high share of Direct Sales reflects effective relationships between manufacturers and healthcare providers.
Gastrointestinal Gi Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Gastrointestinal Gi Stent Industry
Medtronic :
Medtronic is a global leader in medical technology, providing innovative solutions for gastrointestinal issues through advanced stenting technologies.Boston Scientific:
Boston Scientific is known for its therapeutic solutions and comprehensive product portfolio, which includes a wide range of gastrointestinal stents designed for a variety of conditions.Cook Medical:
Cook Medical specializes in gastrointestinal devices and employs state-of-the-art manufacturing techniques to create reliable and effective stenting solutions.Johnson & Johnson:
Johnson & Johnson has a strong presence in the gastrointestinal market, offering innovative stent designs and advancing minimally invasive procedures.Abbott Laboratories:
Abbott Laboratories focuses on innovation within the healthcare sector, providing gastrointestinal stents that enhance treatment efficacy and patient safety.We're grateful to work with incredible clients.









FAQs
What is the market size of gastrointestinal Gi Stent?
The gastrointestinal GI stent market is valued at approximately $3.2 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.7% over the next decade, reaching significant growth by 2033.
What are the key market players or companies in this gastrointestinal Gi Stent industry?
Key players in the gastrointestinal GI stent market include major medical device manufacturers and healthcare companies specializing in gastroenterology innovations. These players drive competition and technological advancements.
What are the primary factors driving the growth in the gastrointestinal Gi Stent industry?
Growth in the gastrointestinal GI stent market is propelled by the increasing prevalence of gastrointestinal diseases, advancements in stent technology, and rising demand for minimally invasive procedures.
Which region is the fastest Growing in the gastrointestinal Gi Stent?
The Asia Pacific region is the fastest-growing market for gastrointestinal GI stents, expecting growth from $0.63 billion in 2023 to $1.23 billion by 2033.
Does ConsaInsights provide customized market report data for the gastrointestinal Gi Stent industry?
Yes, ConsaInsights offers customized market reports tailored to specific client needs within the gastrointestinal GI stent industry, ensuring relevant and precise data.
What deliverables can I expect from this gastrointestinal Gi Stent market research project?
Deliverables typically include comprehensive market analysis, regional insights, segmentation data, growth forecasts, and strategic recommendations for stakeholders in the gastrointestinal GI stent market.
What are the market trends of gastrointestinal Gi Stent?
Key trends in the gastrointestinal GI stent market include advanced materials for stents, an increasing trend toward outpatient procedures, and innovations in stent designs enhancing functionality and patient outcomes.