Gene Therapy Market Report
Published Date: 31 January 2026 | Report Code: gene-therapy
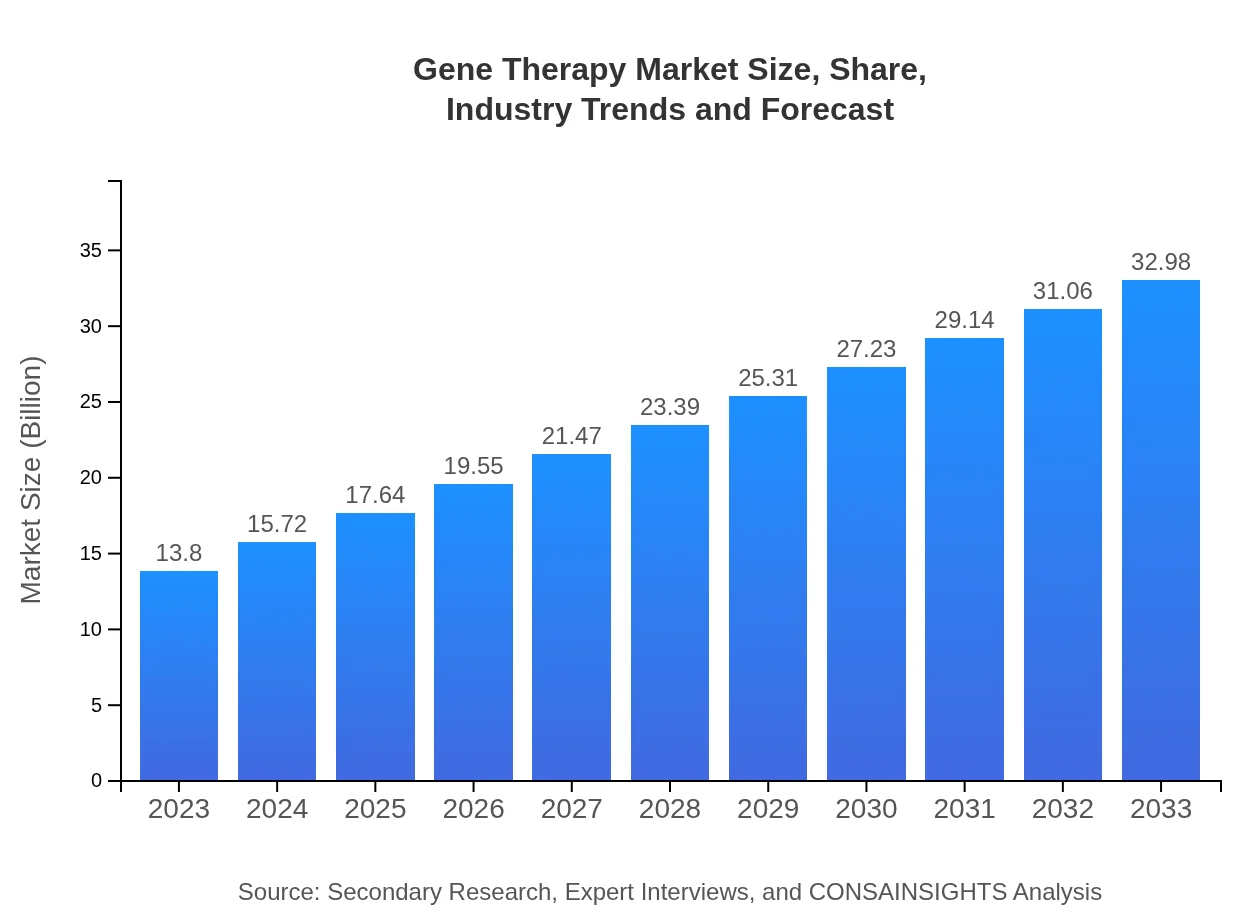
Gene Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Gene Therapy market from 2023 to 2033, highlighting market trends, size, segments, regional insights, and forecasts for growth, alongside current competitive dynamics within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $13.80 Billion |
| CAGR (2023-2033) | 8.8% |
| 2033 Market Size | $32.98 Billion |
| Top Companies | Novartis, Gilead Sciences, Spark Therapeutics, Bluebird Bio, Sarepta Therapeutics |
| Last Modified Date | 31 January 2026 |
Gene Therapy Market Overview
Customize Gene Therapy Market Report market research report
- ✔ Get in-depth analysis of Gene Therapy market size, growth, and forecasts.
- ✔ Understand Gene Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Gene Therapy
What is the Market Size & CAGR of Gene Therapy market in 2023?
Gene Therapy Industry Analysis
Gene Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Gene Therapy Market Analysis Report by Region
Europe Gene Therapy Market Report:
Europe's Gene Therapy market is projected to grow from $4.33 billion in 2023 to $10.34 billion by 2033. The region benefits from strong research capabilities, a well-established pharmaceutical industry, and increasing investments in gene therapies by both public and private sectors.Asia Pacific Gene Therapy Market Report:
The Asia Pacific region is witnessing robust growth in the Gene Therapy market, with an estimated size of $2.62 billion in 2023, projected to reach $6.26 billion by 2033. Increased investments in biopharmaceutical research and a growing prevalence of genetic disorders contribute to this growth. Additionally, supportive governmental policies and collaborations among research institutions bolster the market's expansion.North America Gene Therapy Market Report:
North America accounts for one of the largest shares of the Gene Therapy market, valued at approximately $4.90 billion in 2023 and expected to expand to $11.71 billion by 2033. Leading biotechnology firms, extensive R&D infrastructure, and favorable regulatory frameworks significantly drive this region's growth.South America Gene Therapy Market Report:
In South America, the Gene Therapy market has a smaller yet growing footprint, predicted to grow from $0.71 billion in 2023 to $1.69 billion by 2033. This growth is influenced by increase access to advanced medical technologies and rising awareness of gene therapies in treating genetic diseases.Middle East & Africa Gene Therapy Market Report:
The Middle East and Africa market is expected to increase from $1.25 billion in 2023 to $2.98 billion by 2033. Factors contributing to this growth include government initiatives aimed at improving healthcare systems and increasing collaborations in biotechnology research and innovation.Tell us your focus area and get a customized research report.
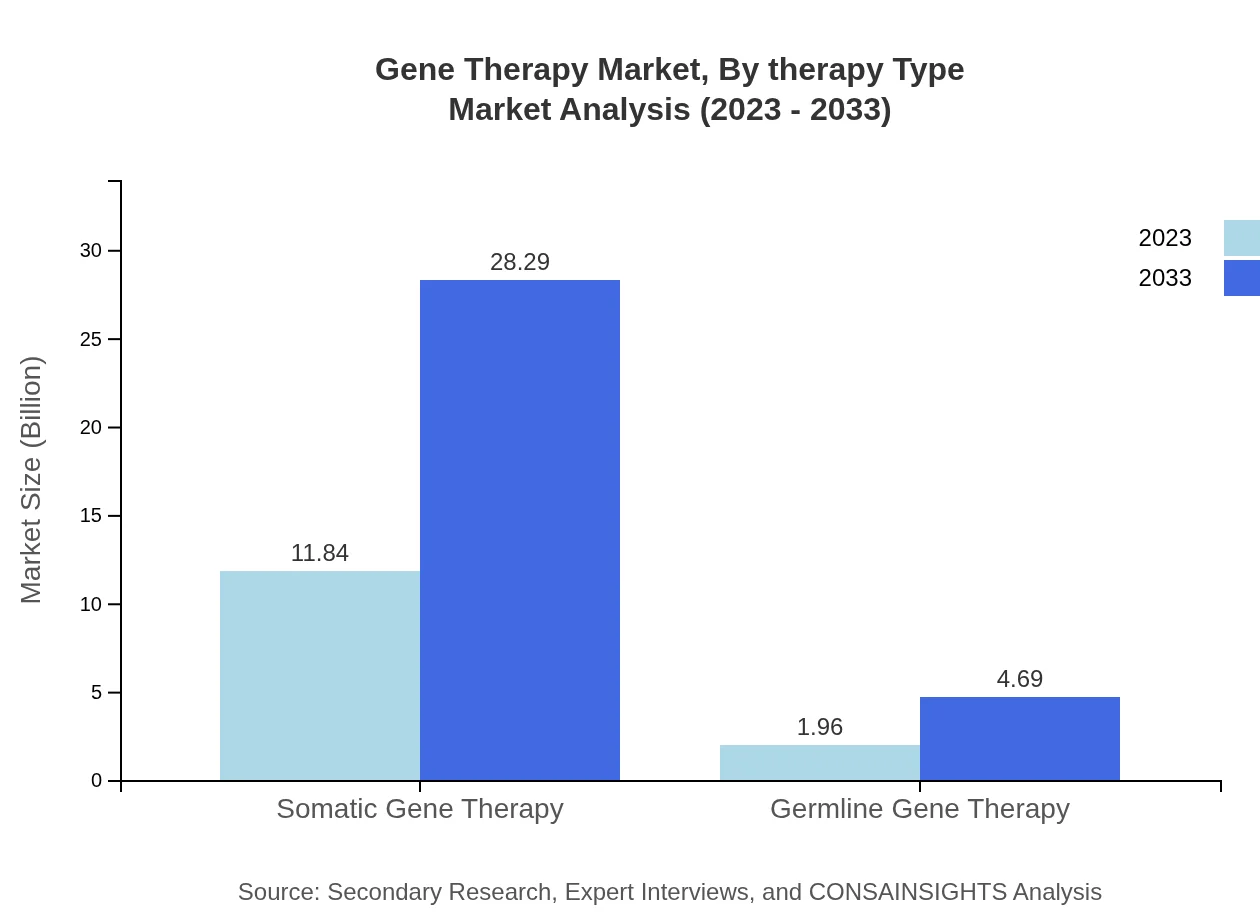
Gene Therapy Market Analysis By Therapy Type
The Gene Therapy market by therapy type is primarily divided into Somatic Gene Therapy and Germline Gene Therapy. Somatic Gene Therapy represents a substantial market share, accounting for $11.84 billion in 2023, projected to grow to $28.29 billion by 2033. Meanwhile, Germline Gene Therapy contributes a smaller share but is still significant, with a market size of $1.96 billion in 2023, projected to reach $4.69 billion by 2033.
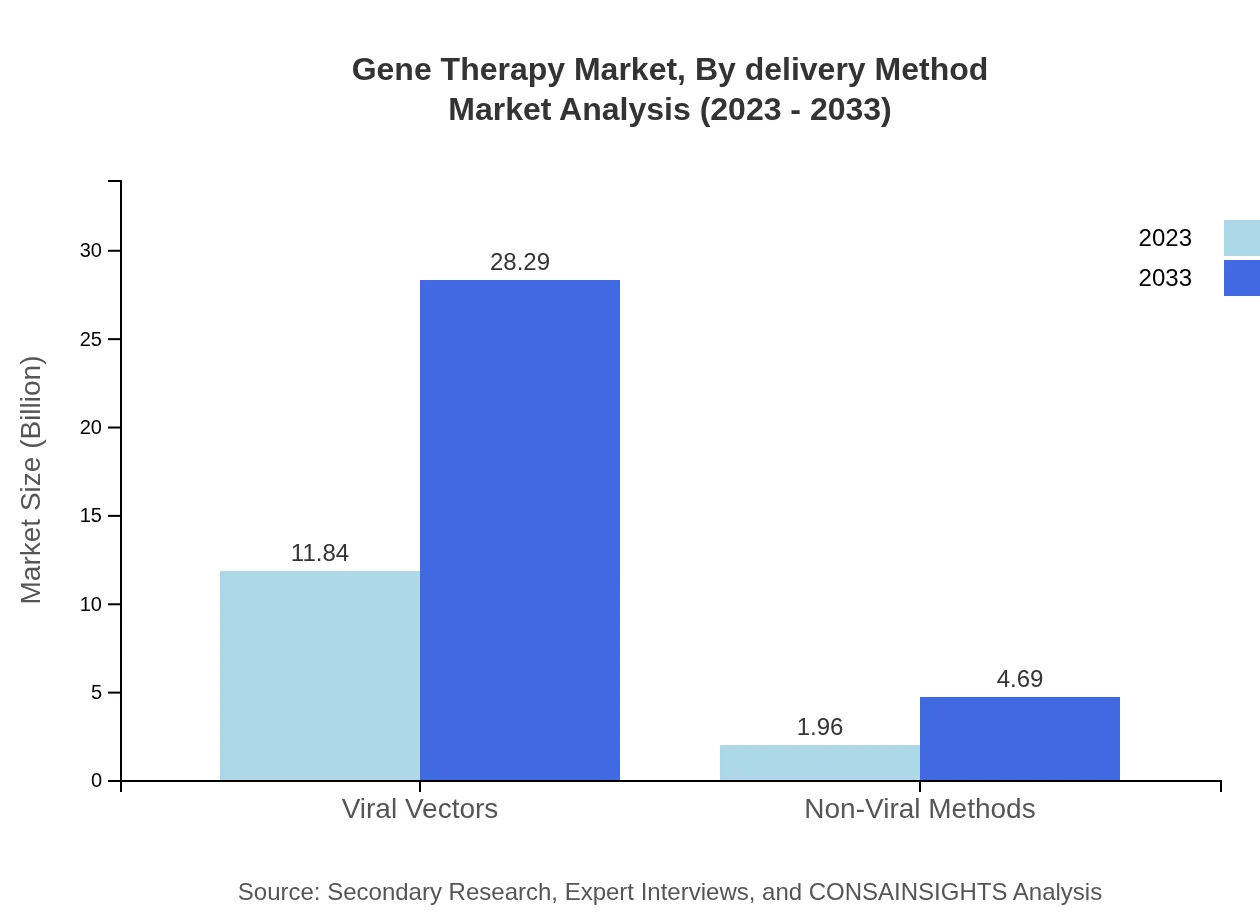
Gene Therapy Market Analysis By Delivery Method
In terms of delivery methods, Viral Vectors dominate the Gene Therapy market, with a size of $11.84 billion expected to rise to $28.29 billion by 2033. Non-Viral Methods, although smaller, indicate growth opportunities, going from $1.96 billion in 2023 to $4.69 billion in 2033, driven by their safety profile and efficiency in gene delivery.
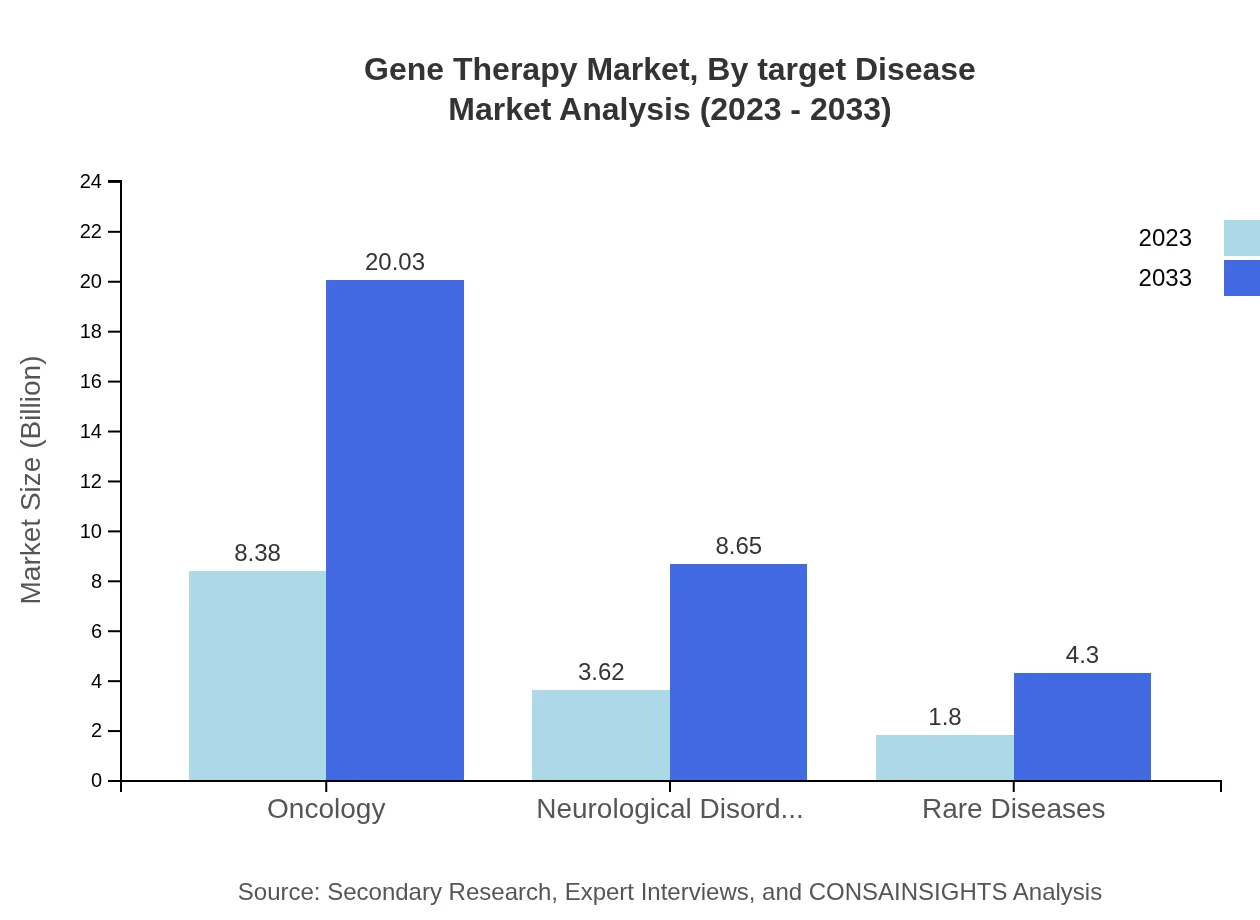
Gene Therapy Market Analysis By Target Disease
Key target diseases in the Gene Therapy market include Oncology, Neurological Disorders, and Rare Diseases. The oncology segment leads with a share of $8.38 billion in 2023, expected to grow to $20.03 billion by 2033. Neurological Disorders and Rare Diseases follow, with projected market sizes of $3.62 billion and $1.80 billion in 2023, respectively.
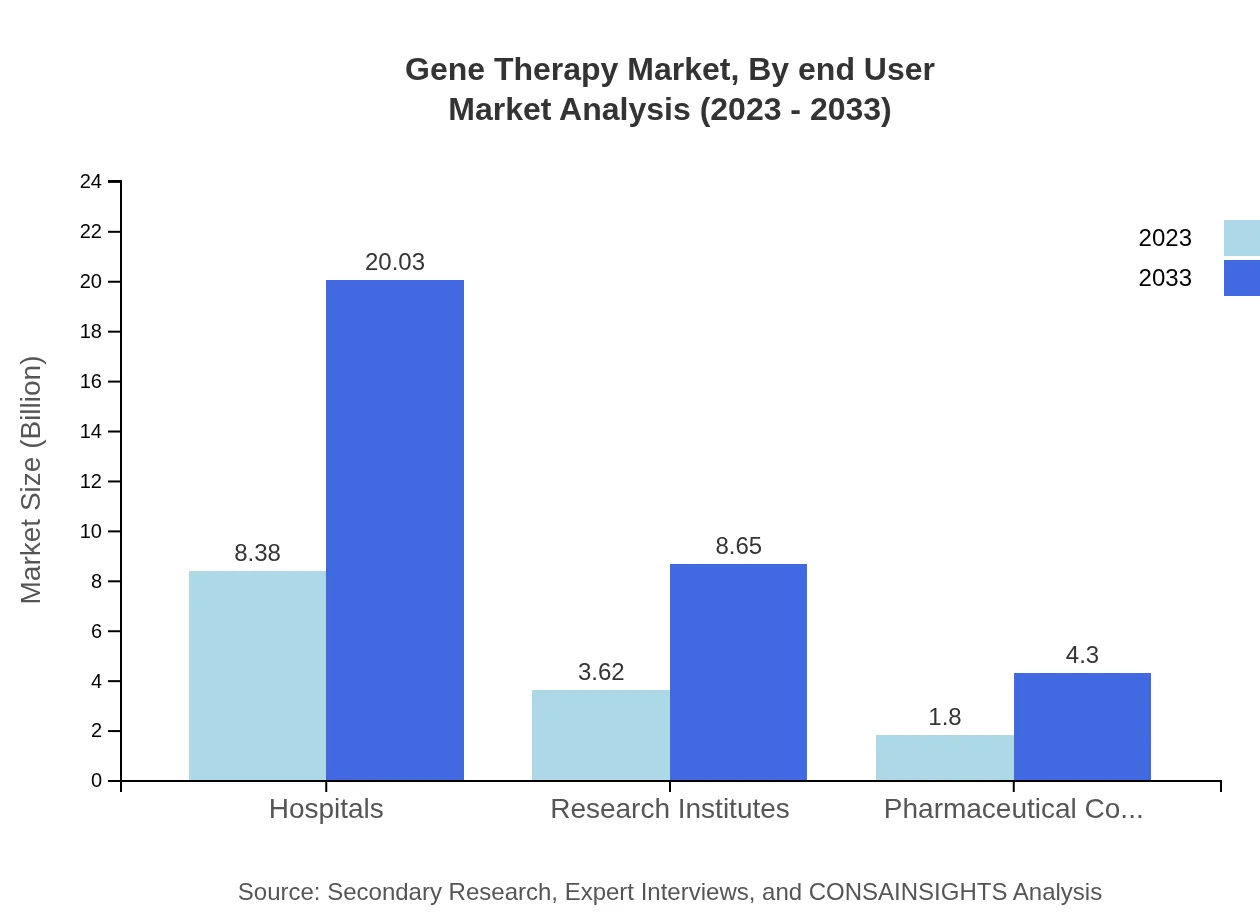
Gene Therapy Market Analysis By End User
The end-users of Gene Therapy include Hospitals, Research Institutes, and Pharmaceutical Companies. Hospitals are expected to maintain the largest share, growing from $8.38 billion in 2023 to $20.03 billion by 2033. Research Institutes and Pharmaceutical Companies will also experience growth, from $3.62 billion and $1.80 billion in 2023, respectively.
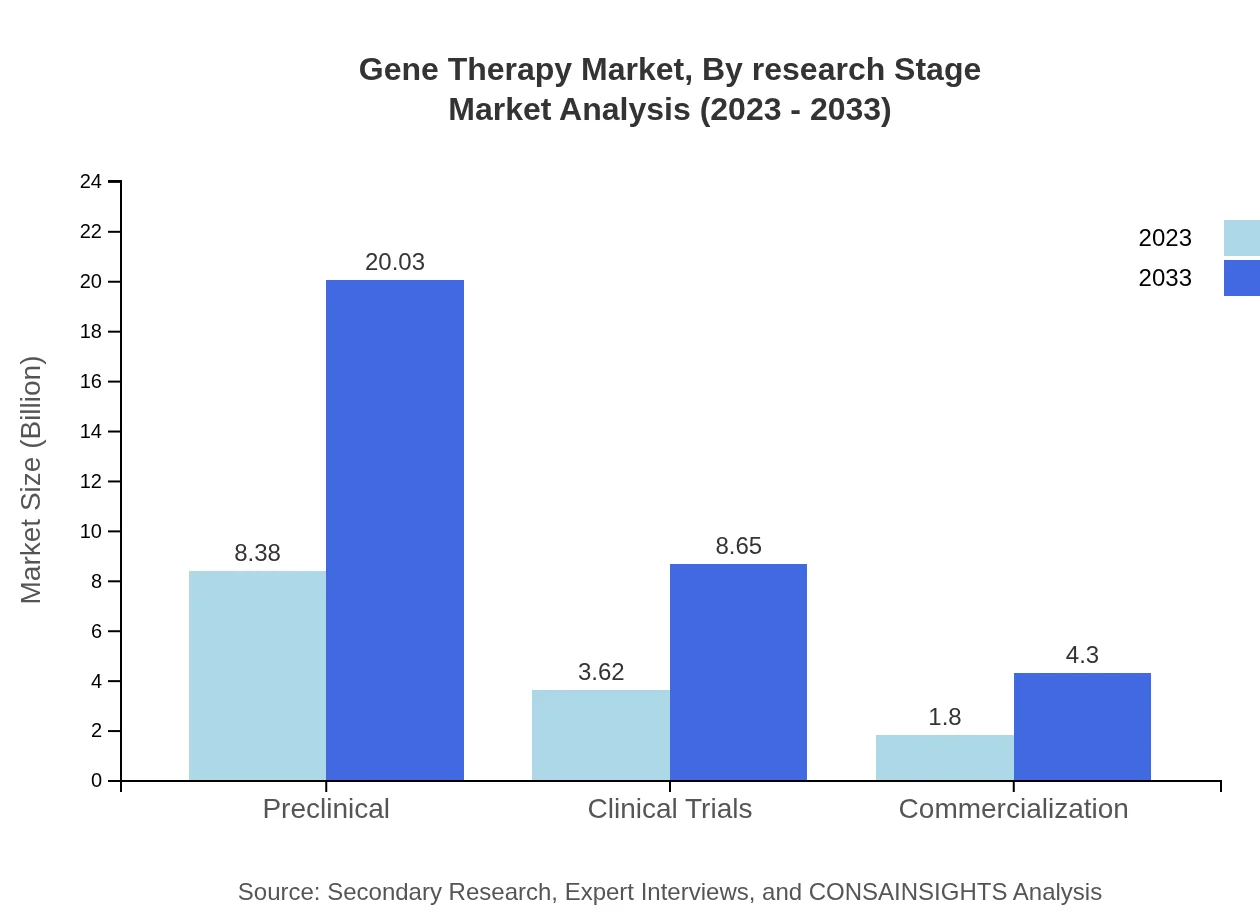
Gene Therapy Market Analysis By Research Stage
Research stages in the Gene Therapy market consist of Preclinical, Clinical Trials, and Commercialization. The Preclinical segment is projected to maintain a significant market size of $8.38 billion in 2023, while Clinical Trials and Commercialization follow with sizes of $3.62 billion and $1.80 billion. Growth is expected across all stages as therapies advance through development.
Gene Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Gene Therapy Industry
Novartis:
A leading pharmaceutical company specializing in innovative therapies including CAR-T cell treatments, setting benchmarks in the Gene Therapy space.Gilead Sciences:
Known for their advancements in antiretroviral therapies, Gilead has also made significant investments in Gene Therapy, particularly for genetic disorders.Spark Therapeutics:
Pioneers in Gene Therapy with a focus on retinal diseases and hemophilia; Spark is known for its innovative approach towards treatment solutions.Bluebird Bio:
This biotechnology company focuses on developing gene therapies for genetic diseases, including sickle cell disease and beta-thalassemia, emphasizing the impact of gene modification.Sarepta Therapeutics:
Specializes in precision genetic medicine and has made significant contributions to therapies for rare diseases, particularly Duchenne muscular dystrophy.We're grateful to work with incredible clients.









FAQs
What is the market size of gene Therapy?
The global gene therapy market is projected to reach approximately $13.8 billion by 2033, growing at a CAGR of 8.8%. This growth is driven by advancements in technology, increased funding, and rising incidences of genetic disorders.
What are the key market players or companies in this gene Therapy industry?
Key players in the gene therapy industry include leading pharmaceutical companies and biotechs specializing in genetic research. They focus on developing innovative therapies targeting a range of diseases, including genetic disorders and cancers.
What are the primary factors driving the growth in the gene therapy industry?
Factors fueling growth in gene therapy include technological advancements in genomic editing, increasing investment in R&D, and heightened awareness of genetic disorders. Additionally, regulatory shifts enhancing approval processes further accelerate market expansion.
Which region is the fastest Growing in gene therapy?
The North American gene therapy market is the fastest-growing region, expected to rise from $4.90 billion in 2023 to $11.71 billion by 2033. Europe and Asia-Pacific also show significant growth prospects, reflecting a global trend.
Does ConsaInsights provide customized market report data for the gene therapy industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the gene therapy industry, ensuring clients receive relevant insights based on unique market demands.
What deliverables can I expect from this gene therapy market research project?
Deliverables from the gene therapy market research project include comprehensive reports detailing market sizing, growth forecasts, competitor analysis, and strategic insights based on regional and segment evaluations.
What are the market trends of gene therapy?
Current trends in gene therapy include increased use of viral vectors, growth in somatic gene therapies, and rise in clinical trials for neurological disorders. The market is shifting towards personalized medicine and more effective treatment modalities.