Genetic Toxicology Testing Market Report
Published Date: 31 January 2026 | Report Code: genetic-toxicology-testing
Genetic Toxicology Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive overview of the Genetic Toxicology Testing market from 2023 to 2033, highlighting market conditions, size, growth projections, and an in-depth analysis of the industry landscape, regional dynamics, and key market players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
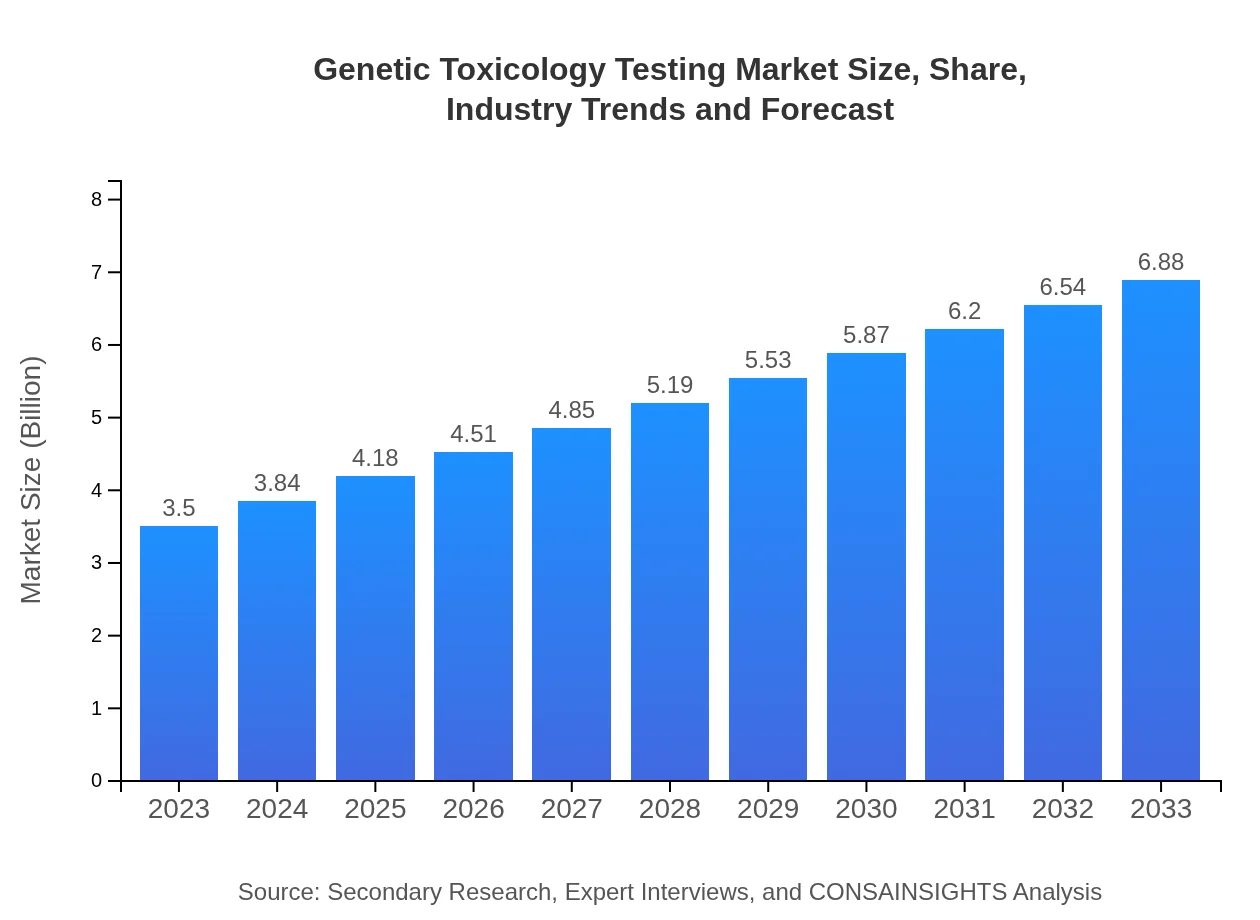
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Charles River Laboratories, SGS, Eurofins Scientific, Covance |
| Last Modified Date | 31 January 2026 |
Genetic Toxicology Testing Market Overview
Customize Genetic Toxicology Testing Market Report market research report
- ✔ Get in-depth analysis of Genetic Toxicology Testing market size, growth, and forecasts.
- ✔ Understand Genetic Toxicology Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Genetic Toxicology Testing
What is the Market Size & CAGR of Genetic Toxicology Testing market in 2023?
Genetic Toxicology Testing Industry Analysis
Genetic Toxicology Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Genetic Toxicology Testing Market Analysis Report by Region
Europe Genetic Toxicology Testing Market Report:
The European market, evaluated at $1.16 billion in 2023, is expected to double by 2033, reaching $2.28 billion. This growth is supported by stringent regulatory demands and an increasing emphasis on genetically toxicology testing within the pharmaceutical and cosmetic industries.Asia Pacific Genetic Toxicology Testing Market Report:
In Asia Pacific, the market is valued at approximately $0.65 billion in 2023, expected to grow to $1.27 billion by 2033. Increasing pharmaceutical developments, expanding research funding, and collaborations between research institutions and industries are driving this resurgence.North America Genetic Toxicology Testing Market Report:
North America leads the Genetic Toxicology Testing market with current estimates of $1.22 billion in 2023 and projections of $2.40 billion by 2033. The region benefits from a robust pharmaceutical sector, stringent regulatory frameworks, and advanced research facilities.South America Genetic Toxicology Testing Market Report:
The South American Genetic Toxicology Testing market was valued at around $0.09 billion in 2023 and is expected to reach about $0.17 billion by 2033. The market is poised for growth, driven by an increase in regulatory compliance and investment in biotechnology.Middle East & Africa Genetic Toxicology Testing Market Report:
In the Middle East and Africa, the Genetic Toxicology Testing market is estimated at $0.38 billion in 2023, with growth projections reaching $0.76 billion by 2033. The increase is attributed to rising investments in biotechnology and improvements in healthcare infrastructures.Tell us your focus area and get a customized research report.
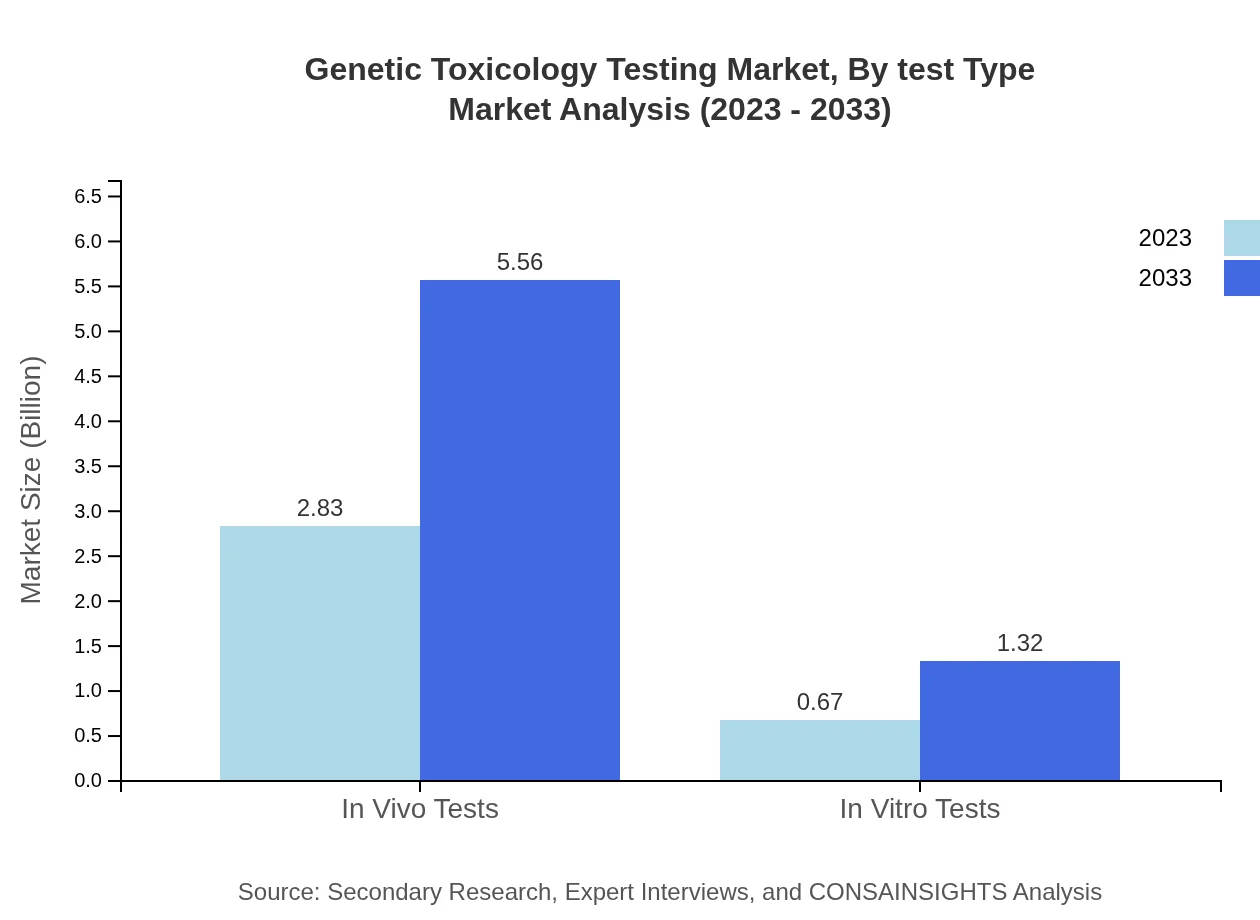
Genetic Toxicology Testing Market Analysis By Test Type
The market based on test type includes significant contributions from both In Vivo Tests, which hold an 80.85% market share in 2023, valued at approximately $2.83 billion, projected to grow to $5.56 billion by 2033; and In Vitro Tests, which, while smaller, show promising growth from $0.67 billion to $1.32 billion over the same period.
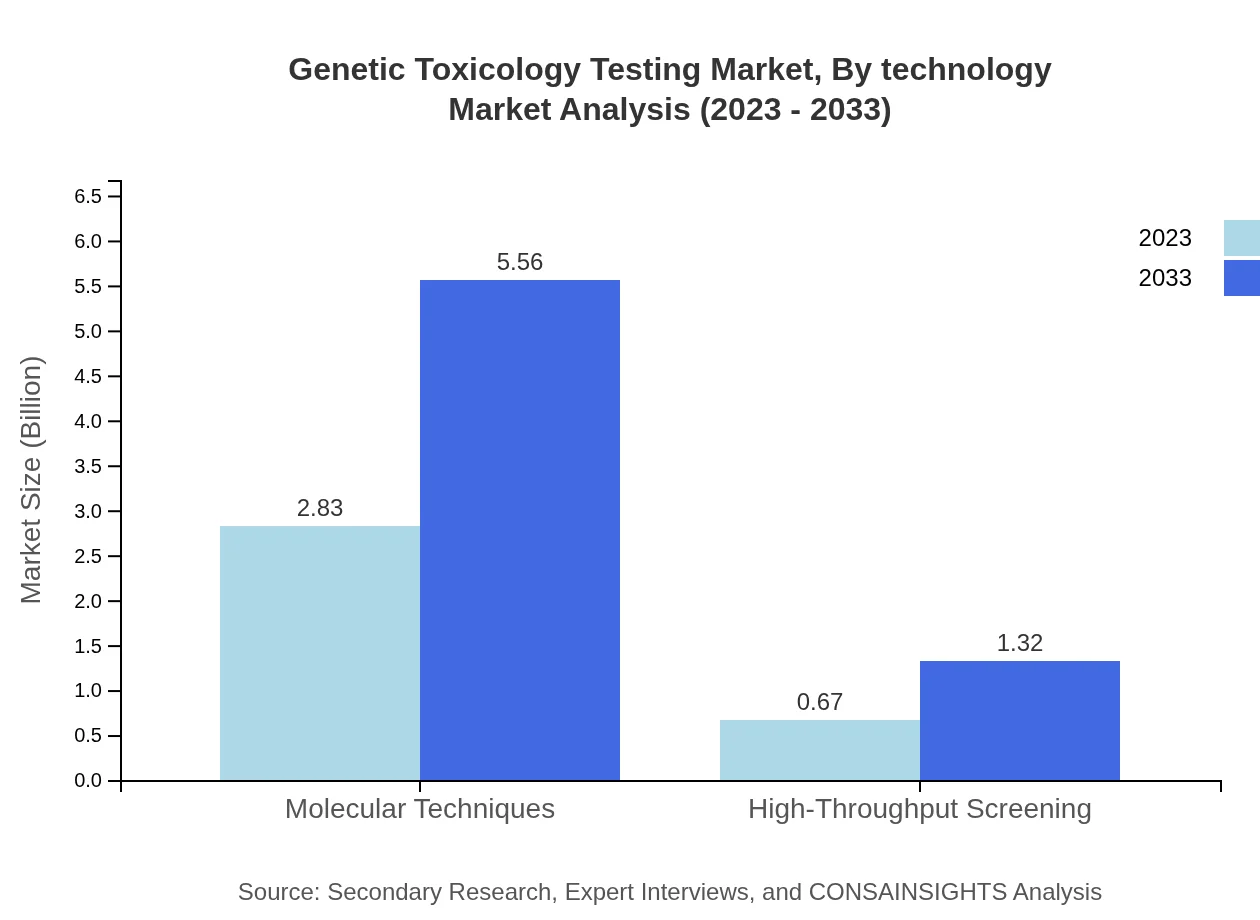
Genetic Toxicology Testing Market Analysis By Technology
This segment highlights the dominance of Molecular Techniques, valued at $2.83 billion in 2023 and anticipated to reach $5.56 billion by 2033, while High-Throughput Screening is projected to grow from $0.67 billion to $1.32 billion, supporting the industry’s push towards more efficient testing methodologies.
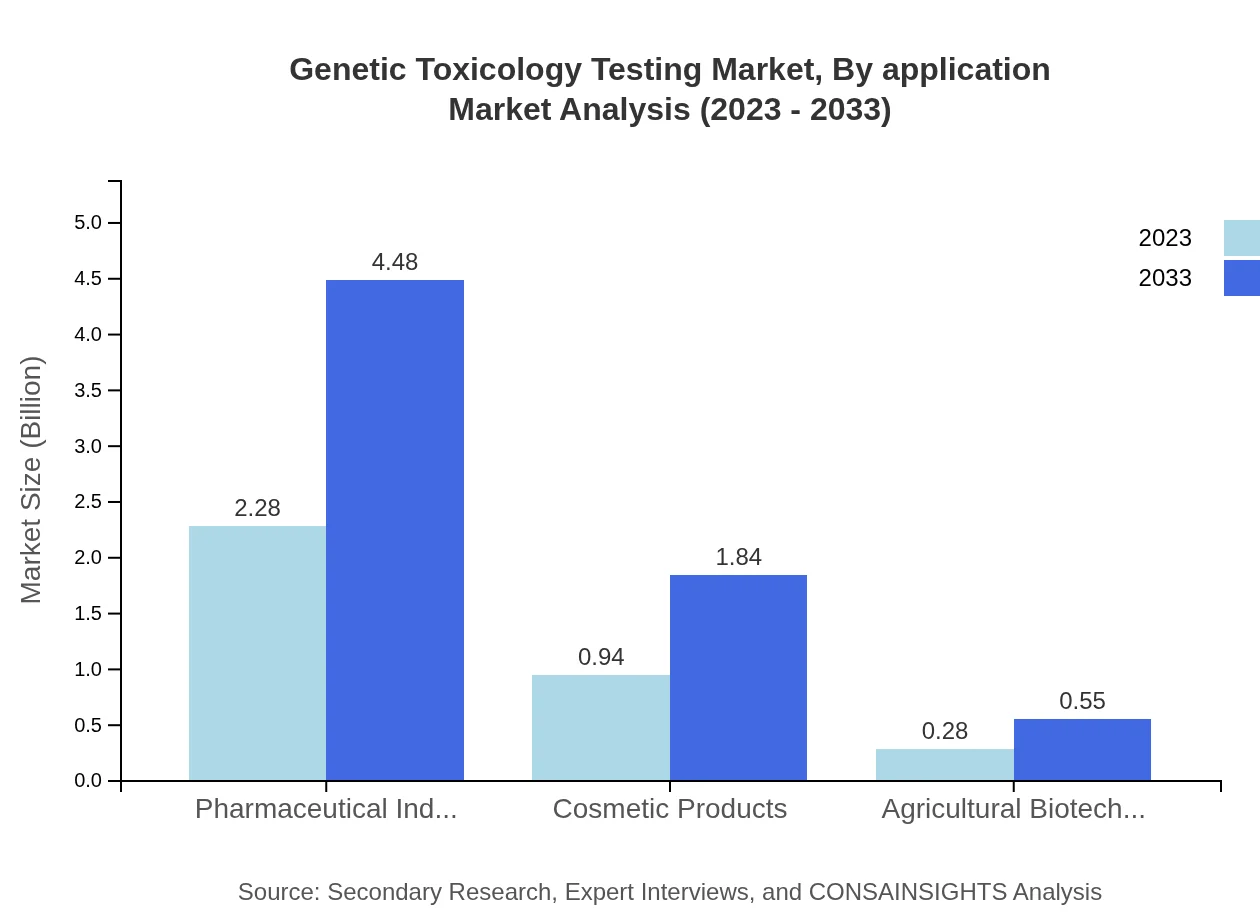
Genetic Toxicology Testing Market Analysis By Application
Within the application segment, the Pharmaceutical Industry represents the largest market share at 65.19% in 2023, reflecting a $2.28 billion valuation and forecasted to reach $4.48 billion by 2033. The Cosmetic Products segment, valued at $0.94 billion in 2023, is expected to grow to $1.84 billion, demonstrating robust demand for safety assessment.
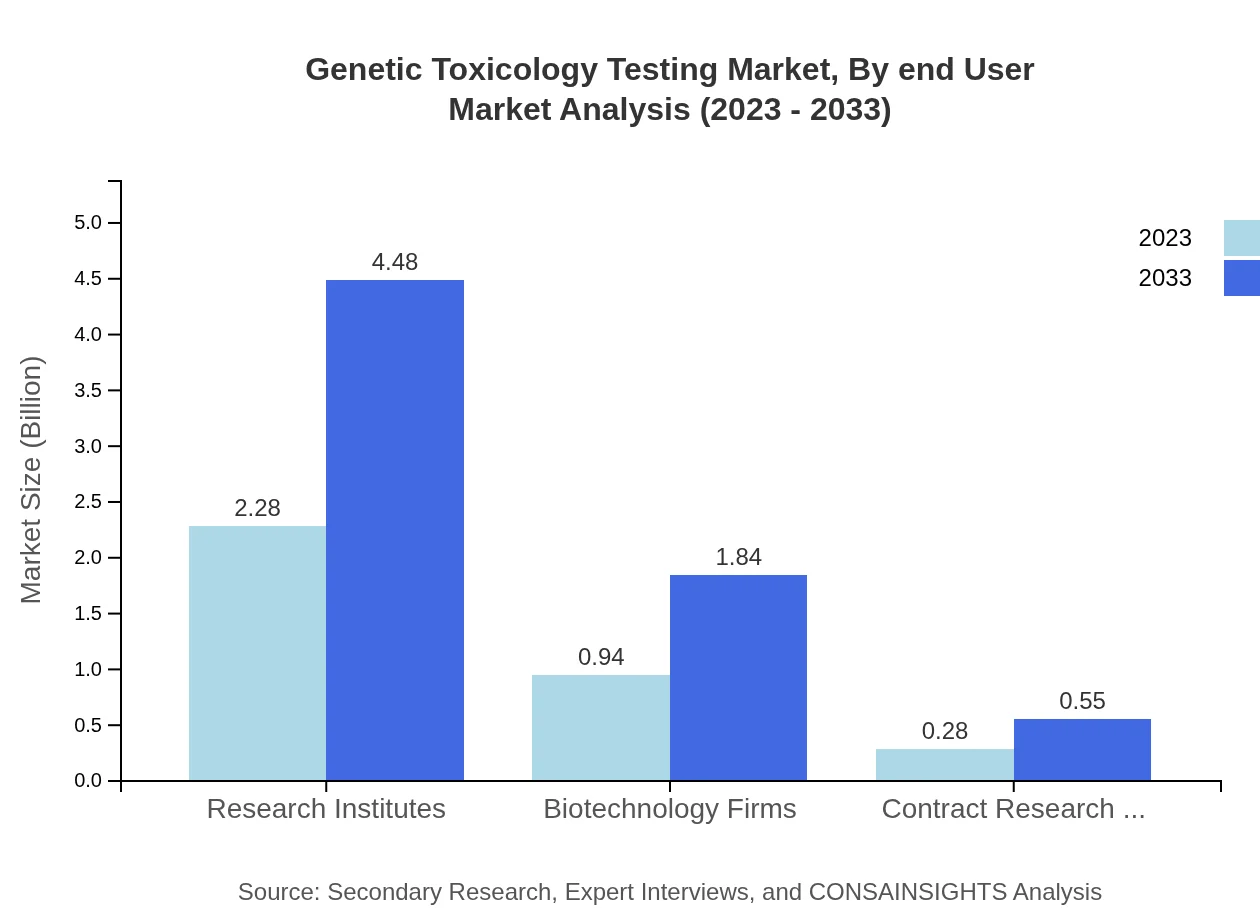
Genetic Toxicology Testing Market Analysis By End User
The primary end-users are Research Institutes, holding a substantial 65.19% share in 2023 at $2.28 billion, projected to grow to $4.48 billion. Biotechnology Firms and Contract Research Organizations (CROs) serve as vital collaborators, accounting for $0.94 billion and $0.28 billion respectively, indicating healthy growth trajectories.
Genetic Toxicology Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Genetic Toxicology Testing Industry
Charles River Laboratories:
A leader in providing essential early-stage pharmaceutical services, Charles River offers a comprehensive range of genetic toxicology testing services aimed at improving drug safety and efficacy.SGS:
SGS is a renowned inspection, verification, testing, and certification company offering a full portfolio of genetic toxicology testing solutions tailored to various industries.Eurofins Scientific:
Eurofins offers a vast array of specialized services, including genetic toxicology testing, focusing on ensuring compliance and safety across pharmaceuticals and consumer products.Covance:
Walgreens Boots Alliance's Covance provides scientific expertise in drug development, including pivotal genetic toxicology testing services enhancing drug safety.We're grateful to work with incredible clients.









FAQs
What is the market size of genetic toxicology testing?
The global genetic toxicology testing market is valued at approximately $3.5 billion in 2023, with a projected CAGR of 6.8% from 2023 to 2033, reflecting the increasing demand for safety assessments.
What are the key market players or companies in this genetic toxicology testing industry?
Key players include prominent biotechnology firms, research institutes, and contract research organizations (CROs), all vying for market share in the rapidly evolving genetic toxicology testing landscape.
What are the primary factors driving the growth in the genetic toxicology testing industry?
Growth is driven by rising regulatory requirements, advancements in molecular techniques, increased investments in pharmaceutical research, and a growing focus on consumer safety, influencing the genetic toxicology testing market positively.
Which region is the fastest Growing in the genetic toxicology testing?
The fastest-growing region is Europe, projected to increase from $1.16 billion in 2023 to $2.28 billion by 2033, influenced by robust regulatory frameworks and research initiatives.
Does ConsaInsights provide customized market report data for the genetic toxicology testing industry?
Yes, ConsaInsights offers customized market reports tailored to the genetic toxicology testing industry, ensuring comprehensive insights that cater to specific client needs.
What deliverables can I expect from this genetic toxicology testing market research project?
Deliverables include detailed market analyses, growth forecasts, segment breakdowns, competitive assessments, and regional insights, providing a comprehensive overview of the genetic toxicology testing landscape.
What are the market trends of genetic toxicology testing?
Current market trends involve increasing use of in vitro testing methods, advancements in high-throughput screening technologies, and a shift towards personalized medicine within the genetic toxicology testing sector.