H1n1 Vaccines Market Report
Published Date: 31 January 2026 | Report Code: h1n1-vaccines
H1n1 Vaccines Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the H1N1 vaccines market from 2023 to 2033. It covers market size, growth forecasts, industry trends, regional insights, and leading companies in the sector, offering valuable insights for stakeholders in the healthcare industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
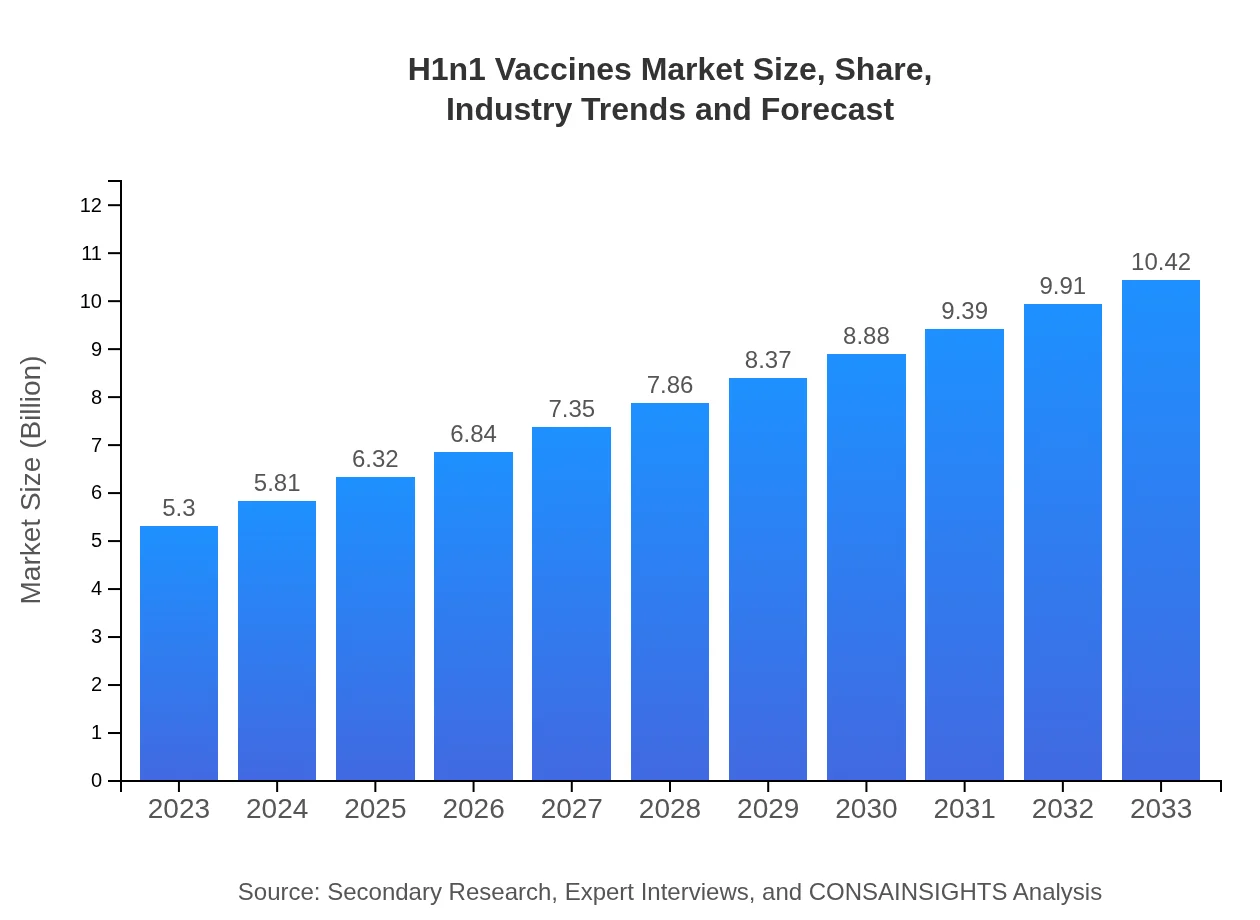
| 2023 Market Size | $5.30 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.42 Billion |
| Top Companies | GlaxoSmithKline, Sanofi Pasteur, Merck & Co., Novartis |
| Last Modified Date | 31 January 2026 |
H1N1 Vaccines Market Overview
Customize H1n1 Vaccines Market Report market research report
- ✔ Get in-depth analysis of H1n1 Vaccines market size, growth, and forecasts.
- ✔ Understand H1n1 Vaccines's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in H1n1 Vaccines
What is the Market Size & CAGR of H1N1 Vaccines market in 2023?
H1N1 Vaccines Industry Analysis
H1N1 Vaccines Market Segmentation and Scope
Tell us your focus area and get a customized research report.
H1N1 Vaccines Market Analysis Report by Region
Europe H1n1 Vaccines Market Report:
The European market for H1N1 vaccines is anticipated to grow from $1.32 billion in 2023 to $2.59 billion by 2033, supported by robust healthcare systems and emphasis on preventive healthcare measures.Asia Pacific H1n1 Vaccines Market Report:
The Asia Pacific region, valued at $1.16 billion in 2023, is projected to grow to $2.29 billion by 2033. This growth is spurred by increasing healthcare expenditure, expanding vaccination programs, and rising public awareness about preventable diseases.North America H1n1 Vaccines Market Report:
North America shows significant growth potential, with the market size projected to increase from $1.72 billion in 2023 to $3.39 billion by 2033. The presence of major pharmaceutical companies and high vaccination coverage are key contributing factors.South America H1n1 Vaccines Market Report:
In South America, the market size for H1N1 vaccines is expected to grow from $0.41 billion in 2023 to $0.81 billion by 2033. Enhanced healthcare infrastructure and government initiatives are driving vaccination campaigns across the region.Middle East & Africa H1n1 Vaccines Market Report:
The Middle East and Africa market is expected to expand from $0.68 billion in 2023 to $1.34 billion by 2033 as a result of improving healthcare infrastructure and rising public health awareness.Tell us your focus area and get a customized research report.
H1n1 Vaccines Market Analysis By Type
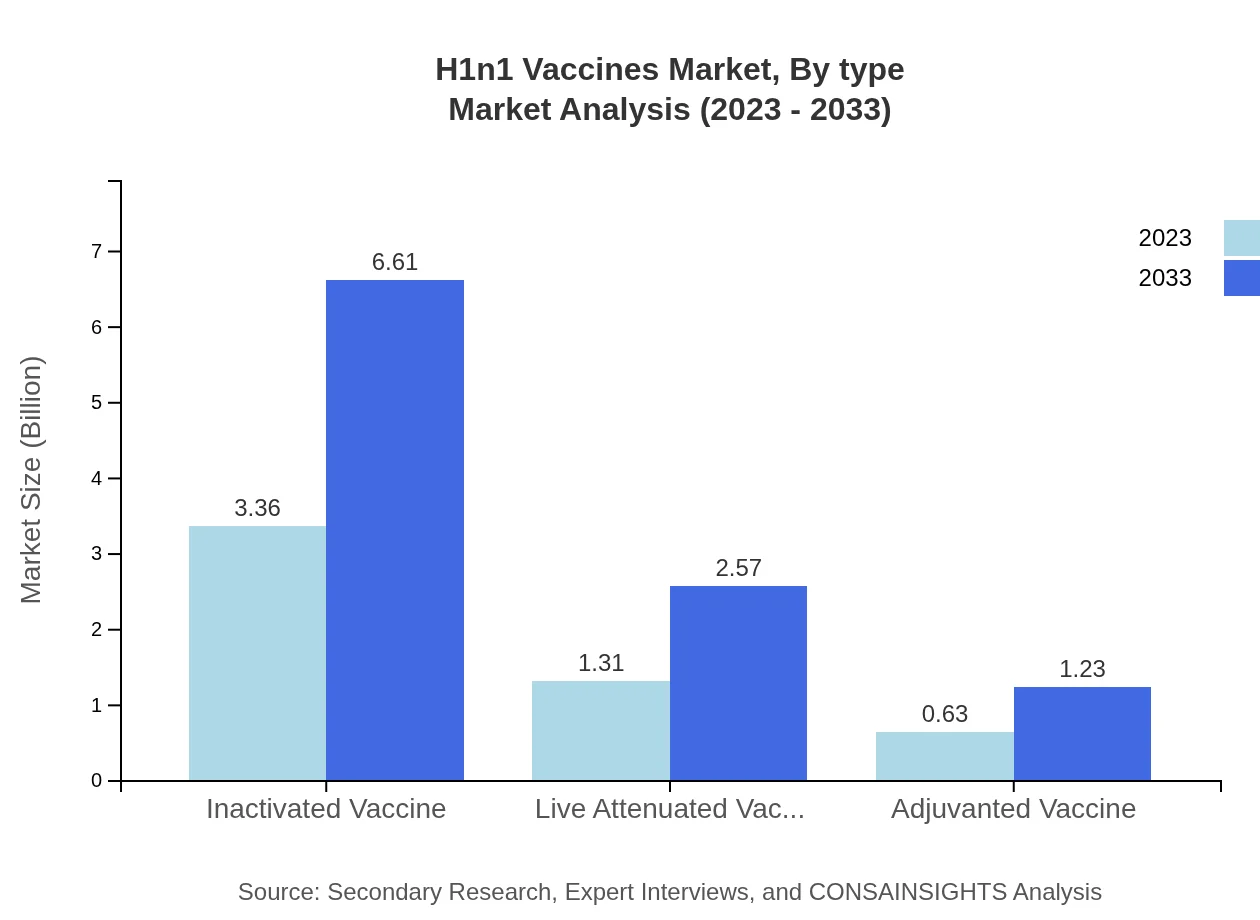
In 2023, the inactivated vaccine segment dominates the market with a size of $3.36 billion, maintaining a market share of 63.49%. This segment is expected to grow to $6.61 billion by 2033. The live attenuated vaccine segment captures a size of $1.31 billion (24.66% share) in 2023, projected to reach $2.57 billion by 2033. Lastly, adjuvanted vaccines, valued at $0.63 billion (11.85% share) in 2023, are expected to grow to $1.23 billion by 2033, reflecting a growing preference for these formulations.
H1n1 Vaccines Market Analysis By Distribution Channel
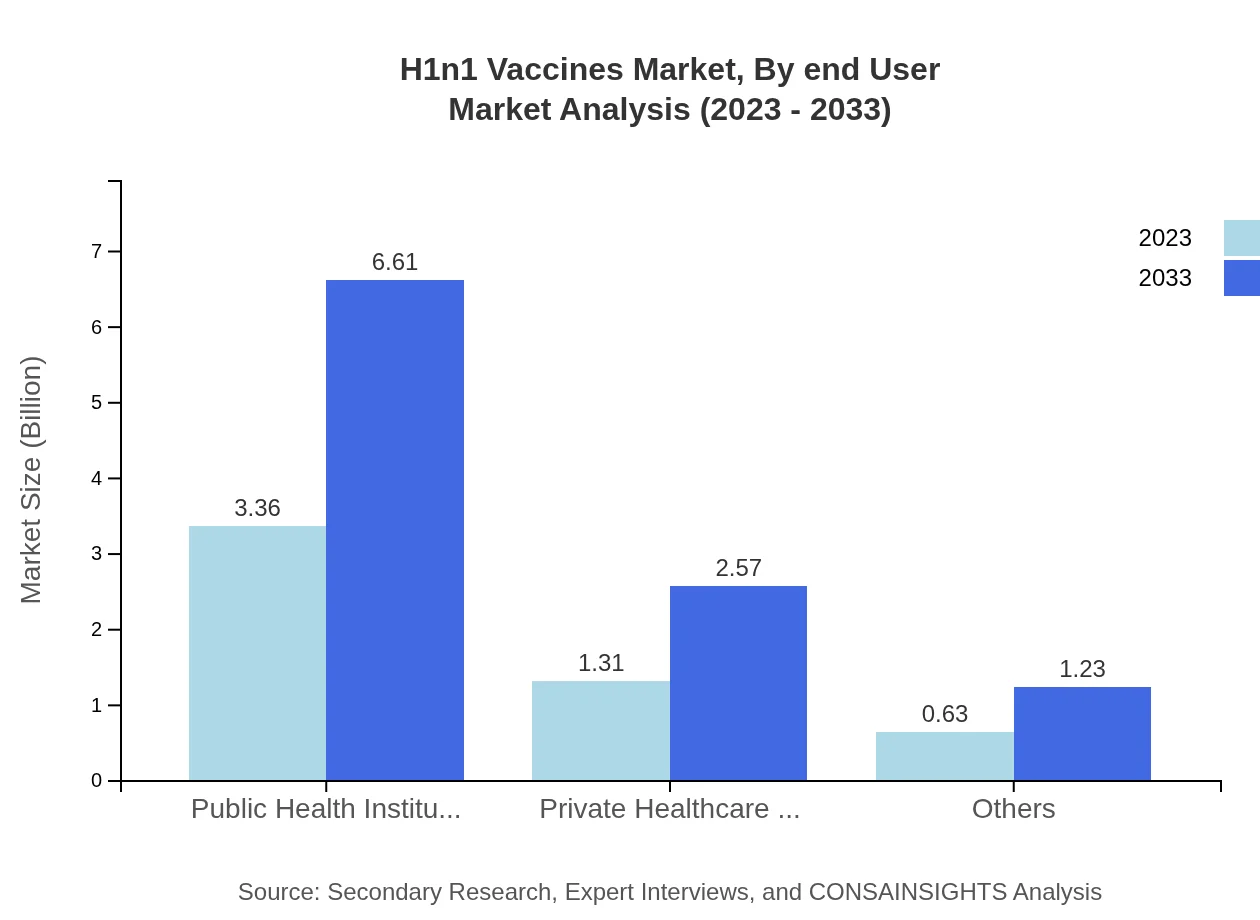
Public health institutions lead with an impressive size of $3.36 billion and a 63.49% market share in 2023. This is expected to grow to $6.61 billion by 2033. Private healthcare facilities follow with a market size of $1.31 billion (24.66% share) in 2023, rising to $2.57 billion by 2033. Pharmacies, clinics, and online pharmacies also contribute significantly, reflecting the diverse channels through which vaccines are distributed.
H1n1 Vaccines Market Analysis By Target Population
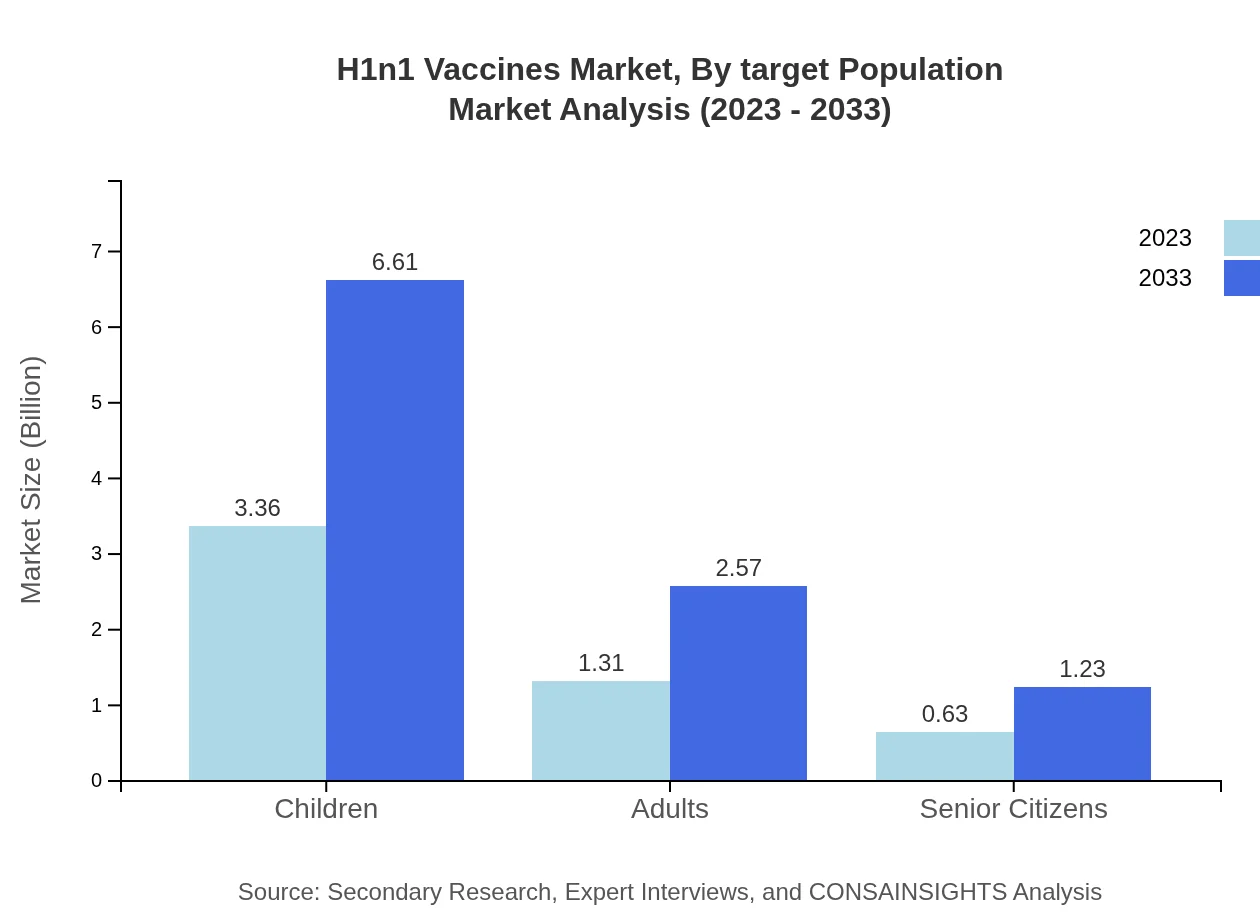
Children form the largest target population segment, with a market size of $3.36 billion (63.49% share) in 2023, anticipated to increase to $6.61 billion by 2033. Adults and senior citizens are also notable segments, valued at $1.31 billion (24.66% share) and $0.63 billion (11.85% share) respectively in 2023, with both segments projected to grow correspondingly.
H1n1 Vaccines Market Analysis By End User
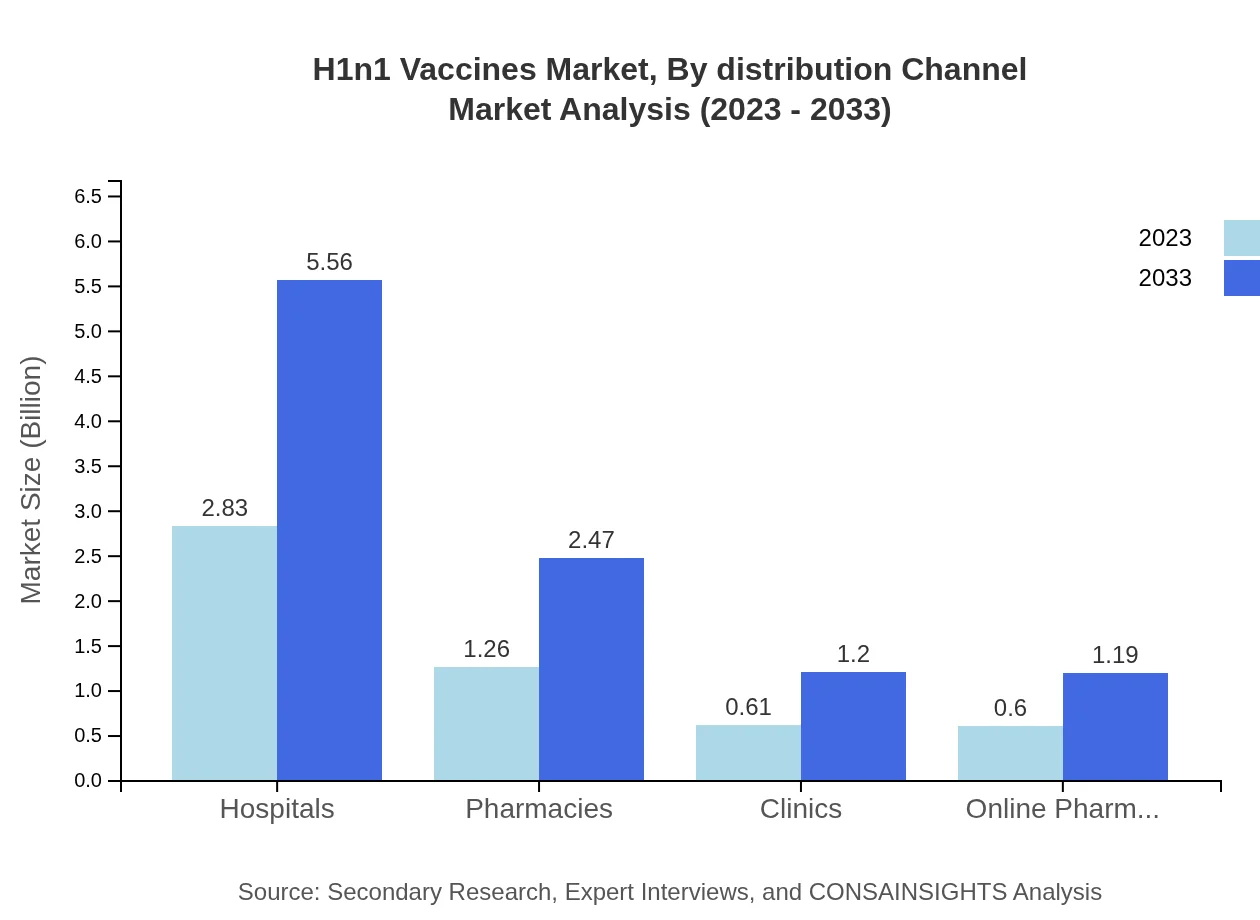
Hospitals account for the largest end-user market, with a size of $2.83 billion (53.4% share) in 2023, projected to reach $5.56 billion by 2033. Pharmacies and clinics contribute substantially, highlighting the crucial role of various healthcare settings in vaccine administration.
H1N1 Vaccines Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in H1N1 Vaccines Industry
GlaxoSmithKline:
One of the largest pharmaceutical companies globally, GSK has made significant contributions to H1N1 vaccine development, focusing on innovative solutions and wide accessibility.Sanofi Pasteur:
A leading vaccine manufacturer, Sanofi Pasteur has been instrumental in producing effective H1N1 vaccines and has a robust global presence.Merck & Co.:
Merck's extensive R&D efforts in vaccine technology have positioned it as a prominent player in the H1N1 vaccine market.Novartis:
Recognized for its innovative vaccines, Novartis plays a key role in addressing the H1N1 influenza outbreak and enhancing vaccine formulations.We're grateful to work with incredible clients.









FAQs
What is the market size of H1N1 Vaccines?
As of 2023, the H1N1 vaccines market is valued at approximately $5.3 billion, with a projected CAGR of 6.8% from 2023 to 2033. This indicates steady growth influenced by ongoing vaccination initiatives and pandemic preparedness.
What are the key market players or companies in the H1N1 Vaccines industry?
Key players in the H1N1 vaccines sector include major pharmaceutical companies such as Sanofi, GlaxoSmithKline, and Merck. These organizations are pivotal for developing, manufacturing, and distributing vaccines globally, enhancing public health measures.
What are the primary factors driving the growth in the H1N1 Vaccines industry?
The growth in the H1N1 vaccines industry is driven by increased awareness of flu prevention, government vaccination programs, innovations in vaccine technology, and responsiveness to pandemic threats. Strong healthcare policies foster growth opportunities.
Which region is the fastest Growing in the H1N1 Vaccines?
North America is the fastest-growing region in the H1N1 vaccines market, expected to grow from $1.72 billion in 2023 to $3.39 billion by 2033, driven by extensive vaccination campaigns and robust healthcare infrastructure.
Does ConsaInsights provide customized market report data for the H1N1 Vaccines industry?
Yes, ConsaInsights offers customized market research reports tailored to specific needs within the H1N1 vaccines industry, allowing clients to access detailed insights suitable for strategic decision-making.
What deliverables can I expect from this H1N1 Vaccines market research project?
Expect comprehensive deliverables including market analysis reports, competitive landscape assessments, growth forecast models, and strategic recommendations tailored to your focus within the H1N1 vaccines market.
What are the market trends of H1N1 Vaccines?
Current trends in the H1N1 vaccines market include increased emphasis on efficacy research, a rise in vaccine accessibility through various channels, and growing public-private partnerships to enhance vaccination coverage and preparedness.