Heart Defect Closure Devices Market Report
Published Date: 31 January 2026 | Report Code: heart-defect-closure-devices
Heart Defect Closure Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Heart Defect Closure Devices market from 2023 to 2033, including insights on market size, growth trends, technological advancements, regional dynamics, and competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
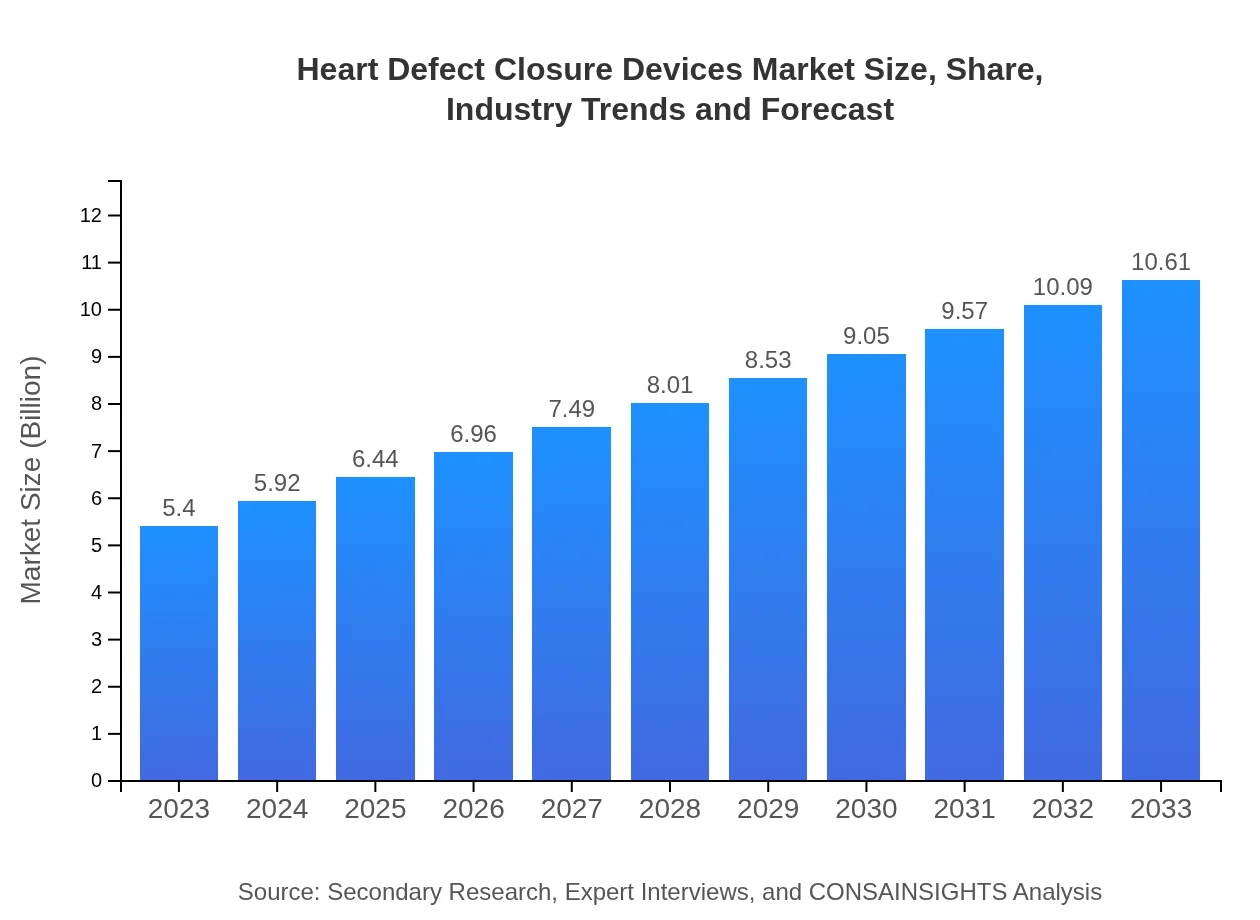
| 2023 Market Size | $5.40 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.61 Billion |
| Top Companies | Abbot Laboratories, Medtronic , Boston Scientific, Edwards Lifesciences, LivaNova |
| Last Modified Date | 31 January 2026 |
Heart Defect Closure Devices Market Overview
Customize Heart Defect Closure Devices Market Report market research report
- ✔ Get in-depth analysis of Heart Defect Closure Devices market size, growth, and forecasts.
- ✔ Understand Heart Defect Closure Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Heart Defect Closure Devices
What is the Market Size & CAGR of Heart Defect Closure Devices market in 2023 and 2033?
Heart Defect Closure Devices Industry Analysis
Heart Defect Closure Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Heart Defect Closure Devices Market Analysis Report by Region
Europe Heart Defect Closure Devices Market Report:
The European market is estimated to grow from USD 1.63 billion in 2023 to USD 3.20 billion by 2033, driven by an aging population and increasing prevalence of heart defects, coupled with substantial research activities in the region.Asia Pacific Heart Defect Closure Devices Market Report:
In the Asia Pacific region, the Heart Defect Closure Devices market is projected to grow from USD 0.98 billion in 2023 to USD 1.93 billion by 2033, reflecting a strong growth rate driven by increasing awareness of congenital heart diseases and improving healthcare infrastructure.North America Heart Defect Closure Devices Market Report:
North America leads the market, with an anticipated increase from USD 2.00 billion in 2023 to USD 3.93 billion by 2033, fueled by high healthcare expenditure, advanced technology adoption, and a robust regulatory framework.South America Heart Defect Closure Devices Market Report:
The South American market is expected to increase from USD 0.22 billion in 2023 to USD 0.43 billion by 2033, supported by rising investment in healthcare and growing adoption of advanced medical devices.Middle East & Africa Heart Defect Closure Devices Market Report:
In the Middle East and Africa, the market is forecasted to rise from USD 0.57 billion in 2023 to USD 1.12 billion by 2033, due to increasing healthcare investments and improving access to advanced medical technologies.Tell us your focus area and get a customized research report.
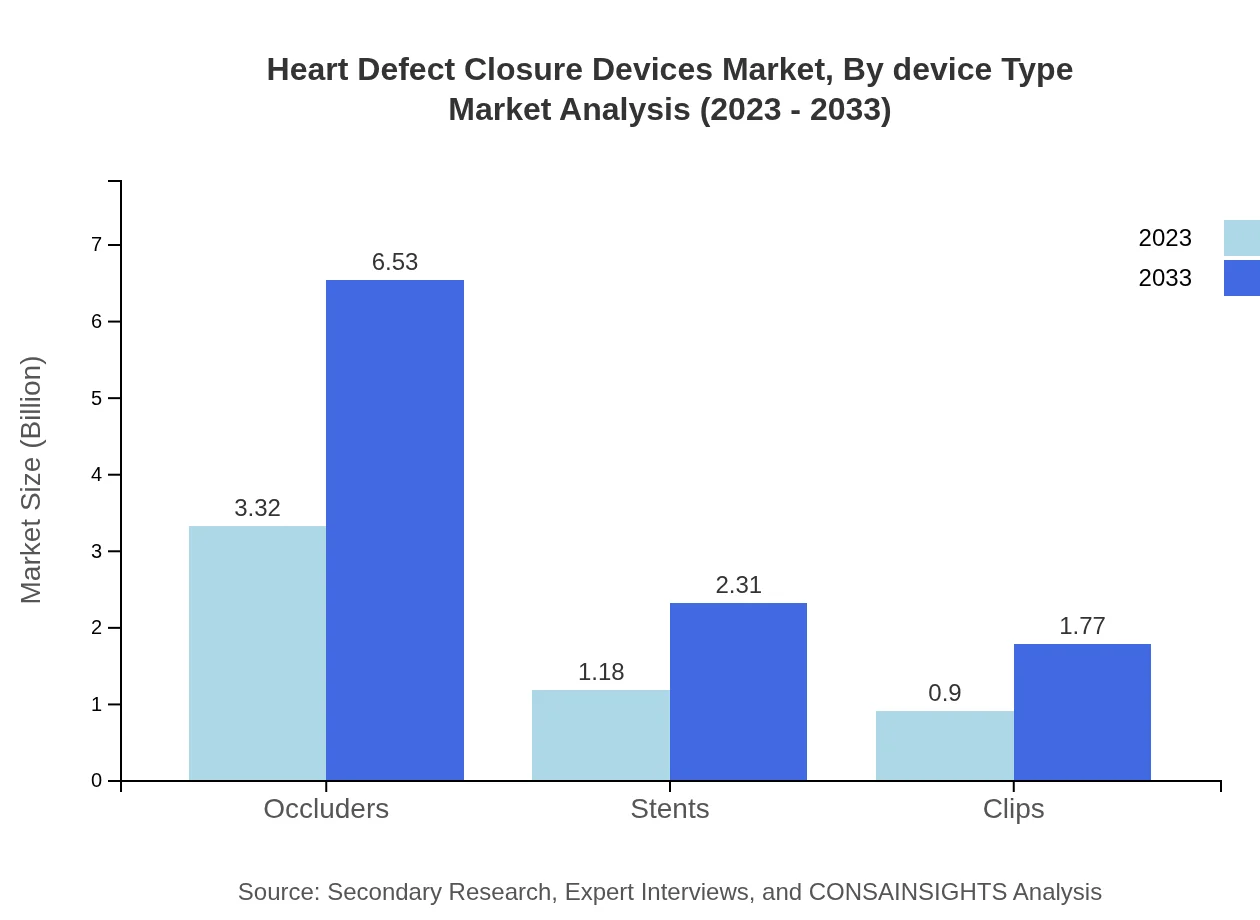
Heart Defect Closure Devices Market Analysis By Device Type
The Heart Defect Closure Devices market by device type includes Occluders, Stents, and Clips. Occluders account for the largest share with a market size of USD 3.32 billion in 2023, expanding to USD 6.53 billion by 2033. Stents and clips follow, showing market sizes of USD 1.18 billion and USD 0.90 billion in 2023, respectively.
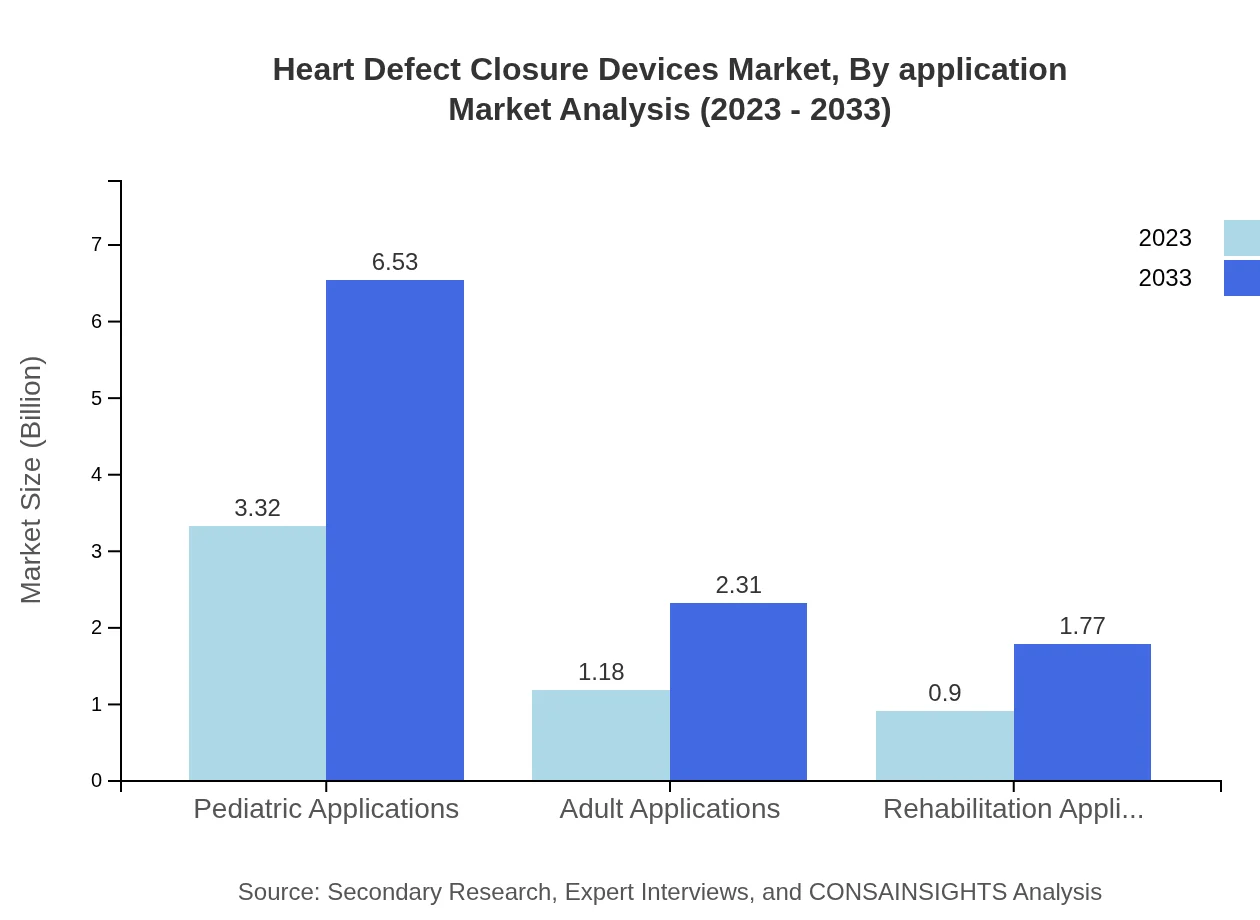
Heart Defect Closure Devices Market Analysis By Application
The market is segmented into Pediatric, Adult, and Rehabilitation applications. Pediatric Applications dominate with a market size of USD 3.32 billion in 2023, expected to grow to USD 6.53 billion by 2033. Adult Applications and Rehabilitation Applications hold sizes of USD 1.18 billion and USD 0.90 billion, respectively, in 2023.
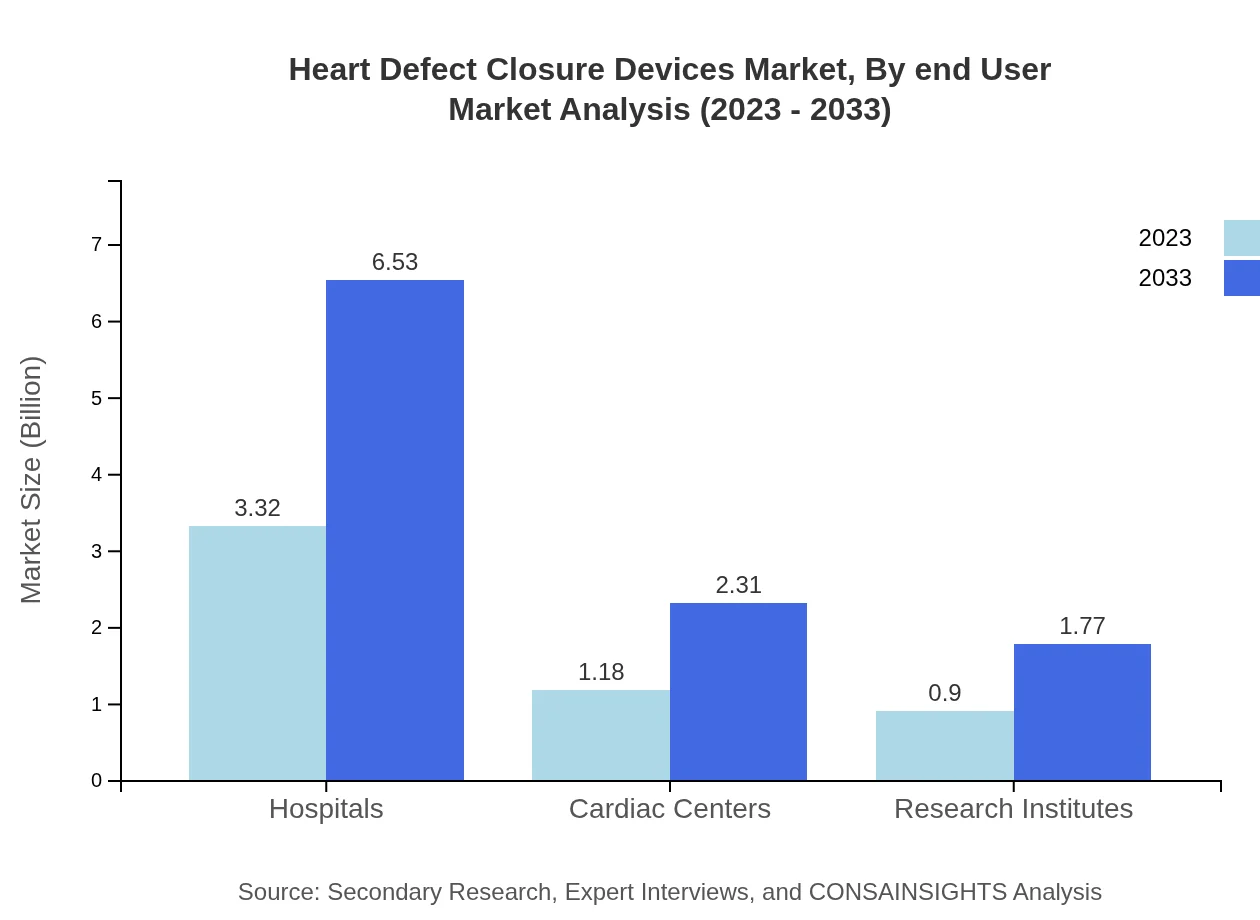
Heart Defect Closure Devices Market Analysis By End User
The primary end-users for Heart Defect Closure Devices include Hospitals, Cardiac Centers, and Research Institutes. Hospitals represent the largest market share with USD 3.32 billion in 2023 and a consistent share of 61.53% throughout the forecast period.
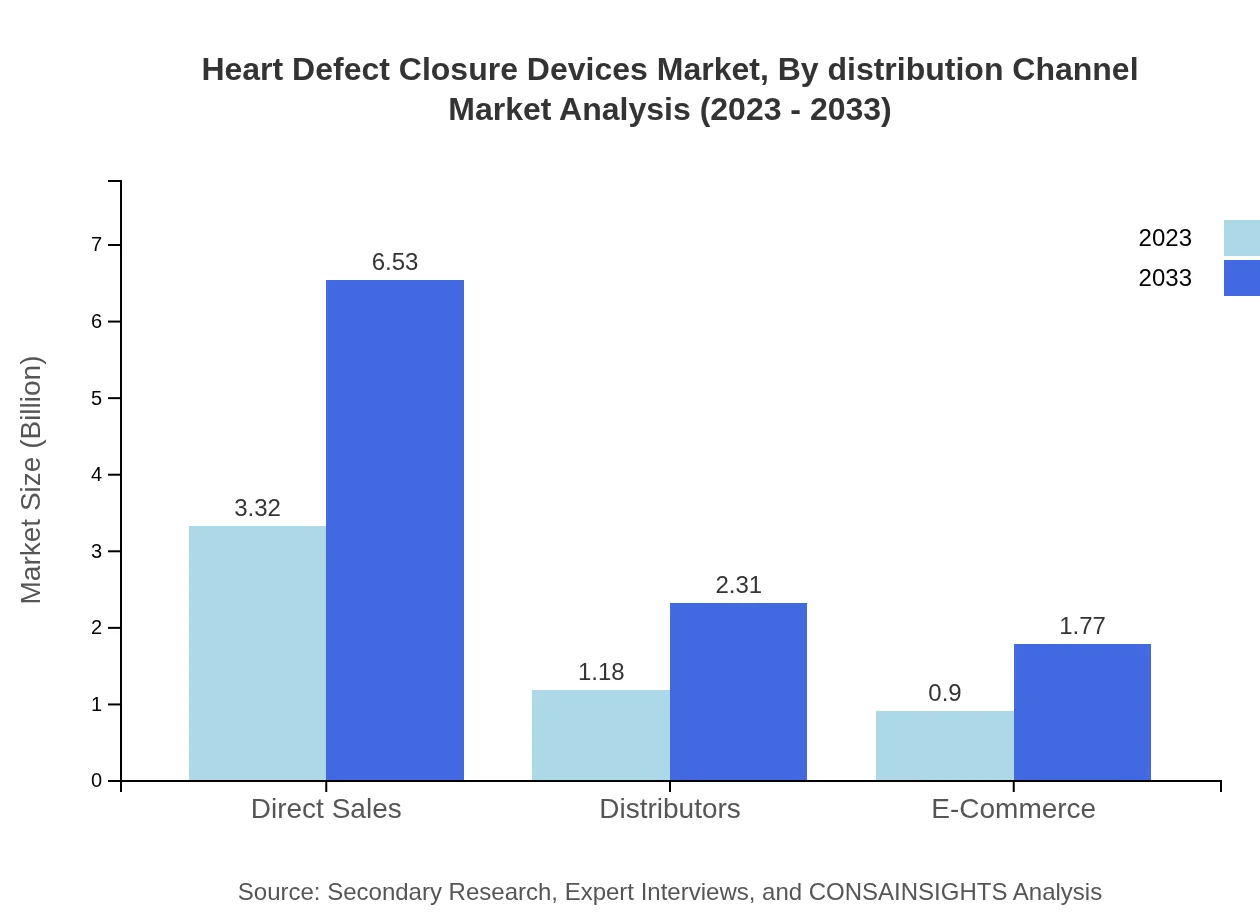
Heart Defect Closure Devices Market Analysis By Distribution Channel
Distribution channels for Heart Defect Closure Devices consist of Direct Sales, Distributors, and E-Commerce. Direct Sales leads with a market size of USD 3.32 billion in 2023, maintaining a 61.53% share, while Distributors and E-Commerce represent 21.77% and 16.7% shares, respectively.
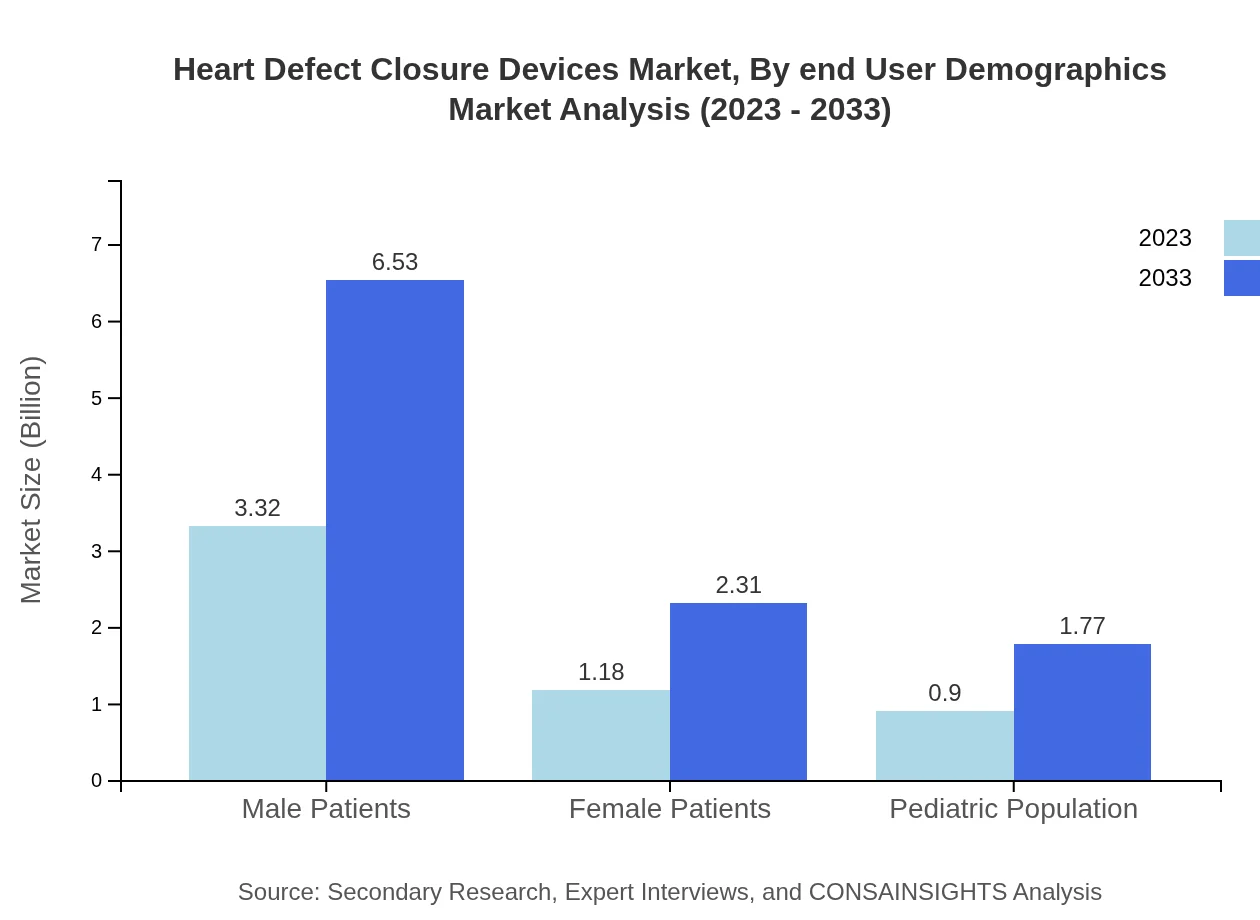
Heart Defect Closure Devices Market Analysis By End User Demographics
End-user demographics categorize the market into Male and Female Patients, as well as the Pediatric Population. Male Patients command the market with sizes of USD 3.32 billion in 2023, while Female Patients and Pediatric Population account for USD 1.18 billion and USD 0.90 billion, respectively.
Heart Defect Closure Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Heart Defect Closure Devices Industry
Abbot Laboratories:
Abbot Laboratories is a leader in medical devices, specializing in heart defect closure devices. Their innovations have significantly advanced closure technologies, improving patient outcomes.Medtronic :
Medtronic is known for its comprehensive range of heart defect closure devices and commitment to research and development, delivering cutting-edge solutions for congenital heart defects.Boston Scientific:
Boston Scientific manufactures a broad portfolio of devices, including highly effective occluders and delivery systems that enhance treatment options for patients with heart defects.Edwards Lifesciences:
Edwards Lifesciences specializes in heart valve therapy and structural heart solutions, contributing to significant advancements in heart defect closure techniques.LivaNova:
LivaNova focuses on innovative solutions in cardiac devices, playing a key role in developing next-generation heart defect closure technologies.We're grateful to work with incredible clients.









FAQs
What is the market size of heart Defect Closure Devices?
The heart defect closure devices market is valued at approximately $5.4 billion in 2023 and is projected to grow at a CAGR of 6.8%, reaching significant market potential by 2033.
What are the key market players or companies in this heart Defect Closure Devices industry?
Key players in the heart defect closure devices market include renowned medical device manufacturers and innovators focusing on cardiac solutions, contributing to significant advancements and market competitiveness.
What are the primary factors driving the growth in the heart Defect Closure Devices industry?
The growth in the heart defect closure devices market is driven by increasing incidences of congenital heart defects, technological advancements in treatment devices, and rising healthcare expenditures globally.
Which region is the fastest Growing in the heart Defect Closure Devices?
Currently, North America holds the largest market share, estimated at $2.00 billion in 2023, while Europe is the fastest-growing region, projected to reach $3.20 billion by 2033.
Does ConsaInsights provide customized market report data for the heart Defect Closure Devices industry?
Yes, Consainsights offers customized market report data tailored specifically for the heart defect closure devices industry, catering to unique client needs for strategic decision-making.
What deliverables can I expect from this heart Defect Closure Devices market research project?
Expect detailed market analysis, regional insights, segmentation data, competitive landscape overviews, and actionable recommendations designed to facilitate informed business strategies.
What are the market trends of heart Defect Closure Devices?
Market trends include increasing adoption of minimally invasive techniques, rising preference for innovative closure devices, and growing applications in pediatric and adult populations, enhancing overall demand.