Helicobacter Pylori Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: helicobacter-pylori-diagnostics
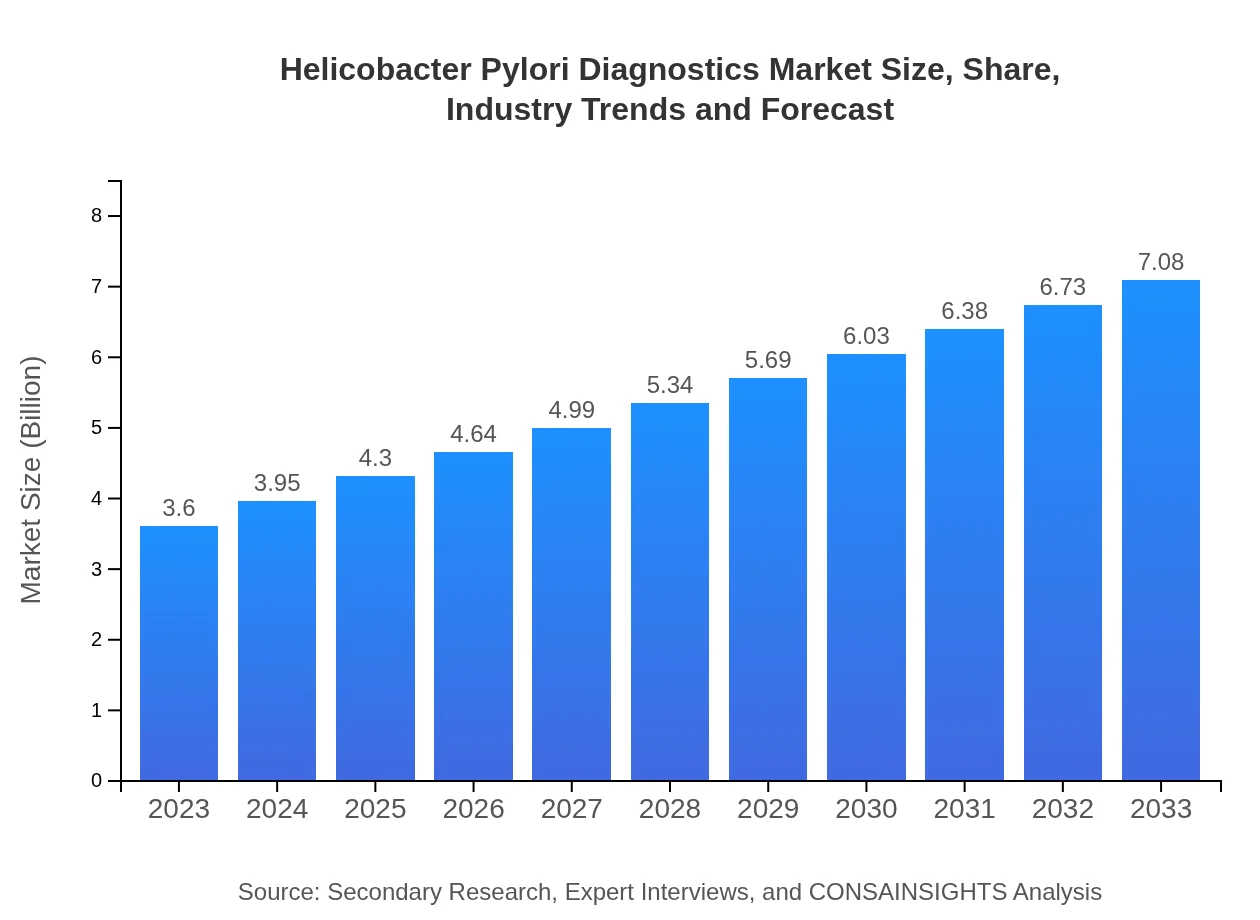
Helicobacter Pylori Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Helicobacter Pylori Diagnostics market from 2023 to 2033, including market overview, size, trends, segmentations, and key players, offering valuable insights for stakeholders in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $7.08 Billion |
| Top Companies | Abbott Laboratories, Thermo Fisher Scientific, Roche Diagnostics, Danaher Corporation |
| Last Modified Date | 31 January 2026 |
Helicobacter Pylori Diagnostics Market Overview
Customize Helicobacter Pylori Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Helicobacter Pylori Diagnostics market size, growth, and forecasts.
- ✔ Understand Helicobacter Pylori Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Helicobacter Pylori Diagnostics
What is the Market Size & CAGR of Helicobacter Pylori Diagnostics market in 2023?
Helicobacter Pylori Diagnostics Industry Analysis
Helicobacter Pylori Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
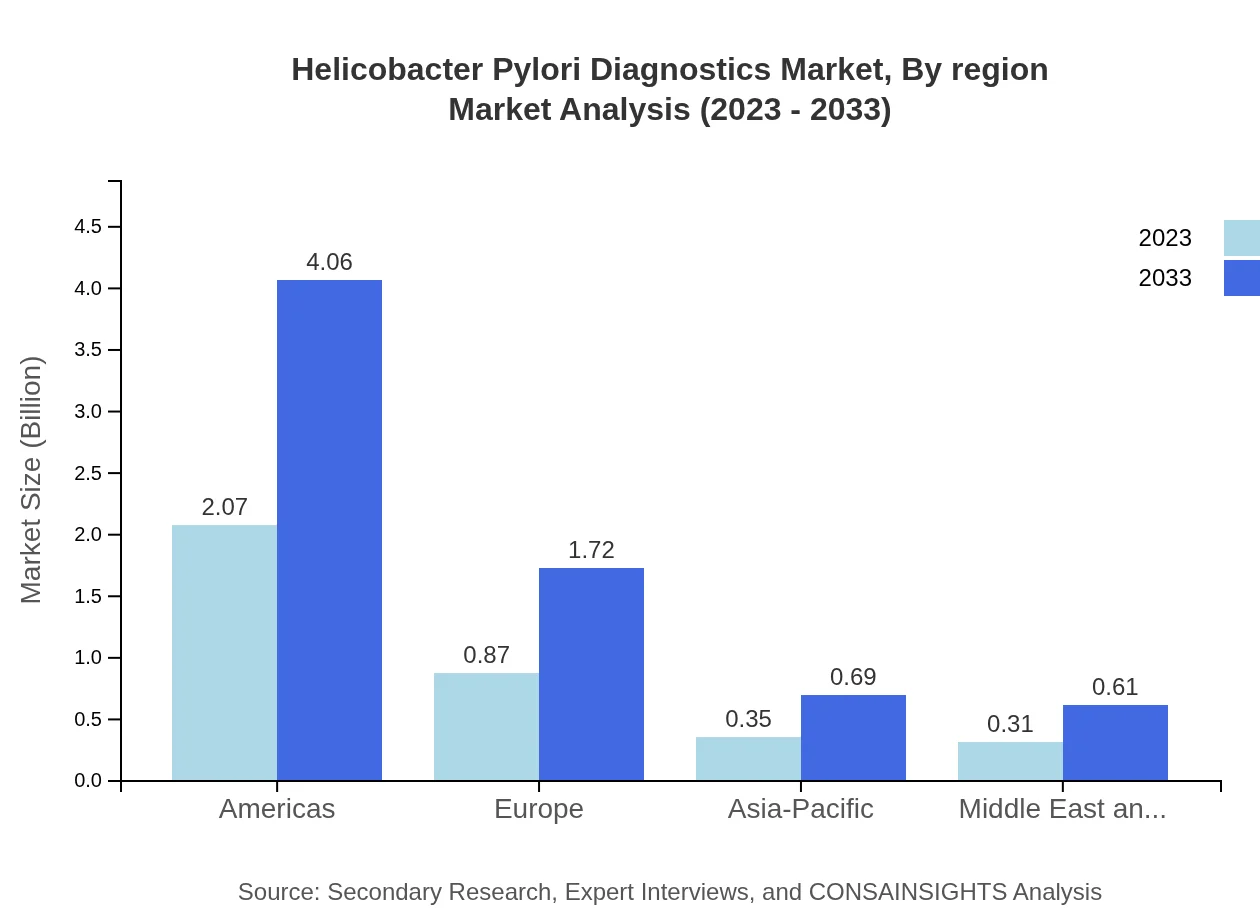
Helicobacter Pylori Diagnostics Market Analysis Report by Region
Europe Helicobacter Pylori Diagnostics Market Report:
Europe is expected to see growth from $1.05 billion in 2023 to $2.06 billion in 2033 with increasing investments in healthcare and more patients seeking diagnostics.Asia Pacific Helicobacter Pylori Diagnostics Market Report:
In the Asia-Pacific region, the Helicobacter Pylori Diagnostics market was valued at $0.66 billion in 2023 and is expected to reach $1.30 billion by 2033, reflecting a growing awareness and access to diagnostic facilities.North America Helicobacter Pylori Diagnostics Market Report:
North America is the largest market, beginning at $1.35 billion in 2023 and projected to grow to $2.66 billion by 2033, driven by advanced healthcare technology and high diagnostic adoption rates.South America Helicobacter Pylori Diagnostics Market Report:
The South American market is smaller but shows significant growth potential, moving from $0.08 billion in 2023 to $0.15 billion by 2033 as healthcare infrastructure improves.Middle East & Africa Helicobacter Pylori Diagnostics Market Report:
The Middle East and Africa market, valued at $0.46 billion in 2023 will grow to approximately $0.90 billion by 2033 as awareness and healthcare investments increase.Tell us your focus area and get a customized research report.
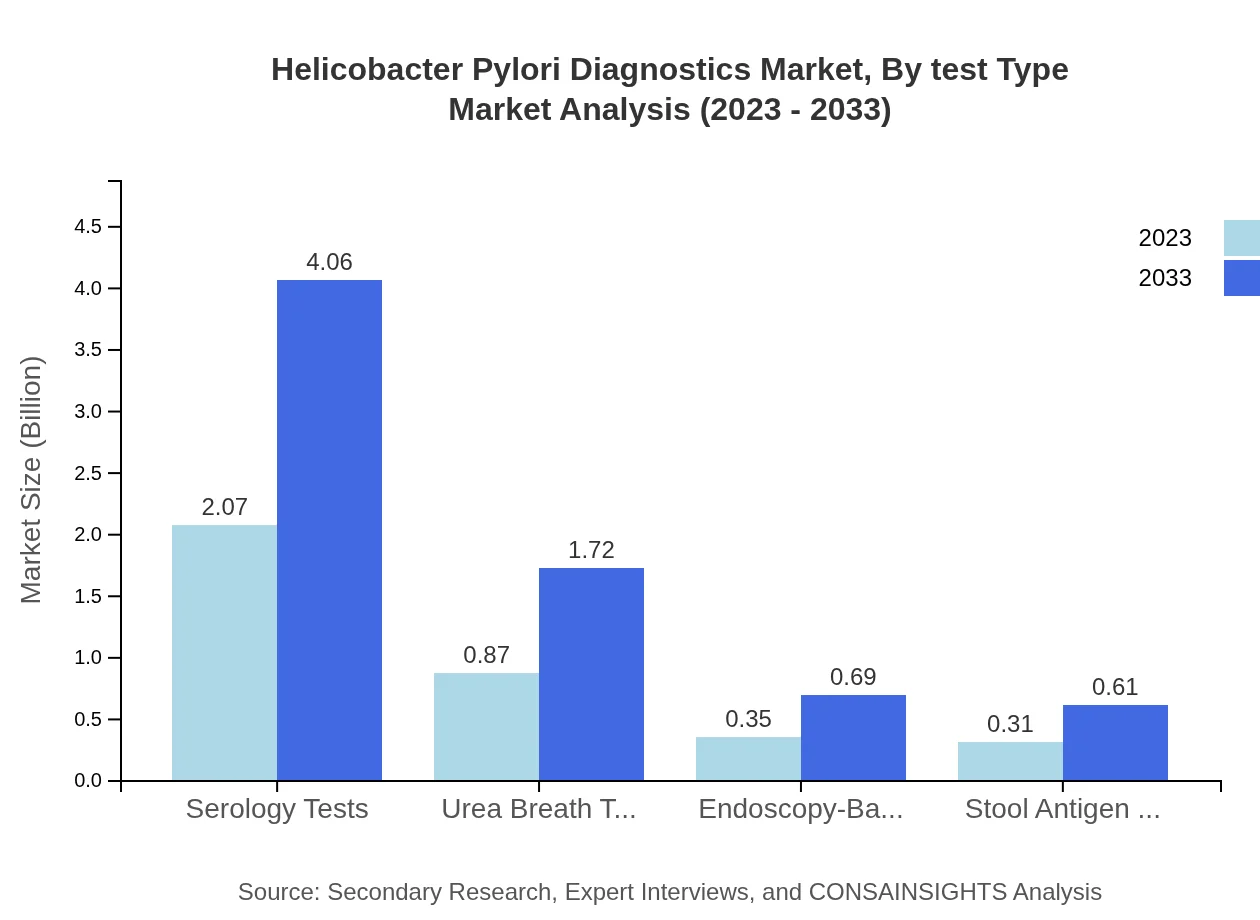
Helicobacter Pylori Diagnostics Market Analysis By Test Type
The dominant segment in the Helicobacter Pylori Diagnostics market is serology tests, expected to grow from $2.07 billion in 2023 to $4.06 billion by 2033. The urea breath tests and stool antigen tests are also significant, demonstrating robust growth and market demand due to their effectiveness and non-invasive nature.
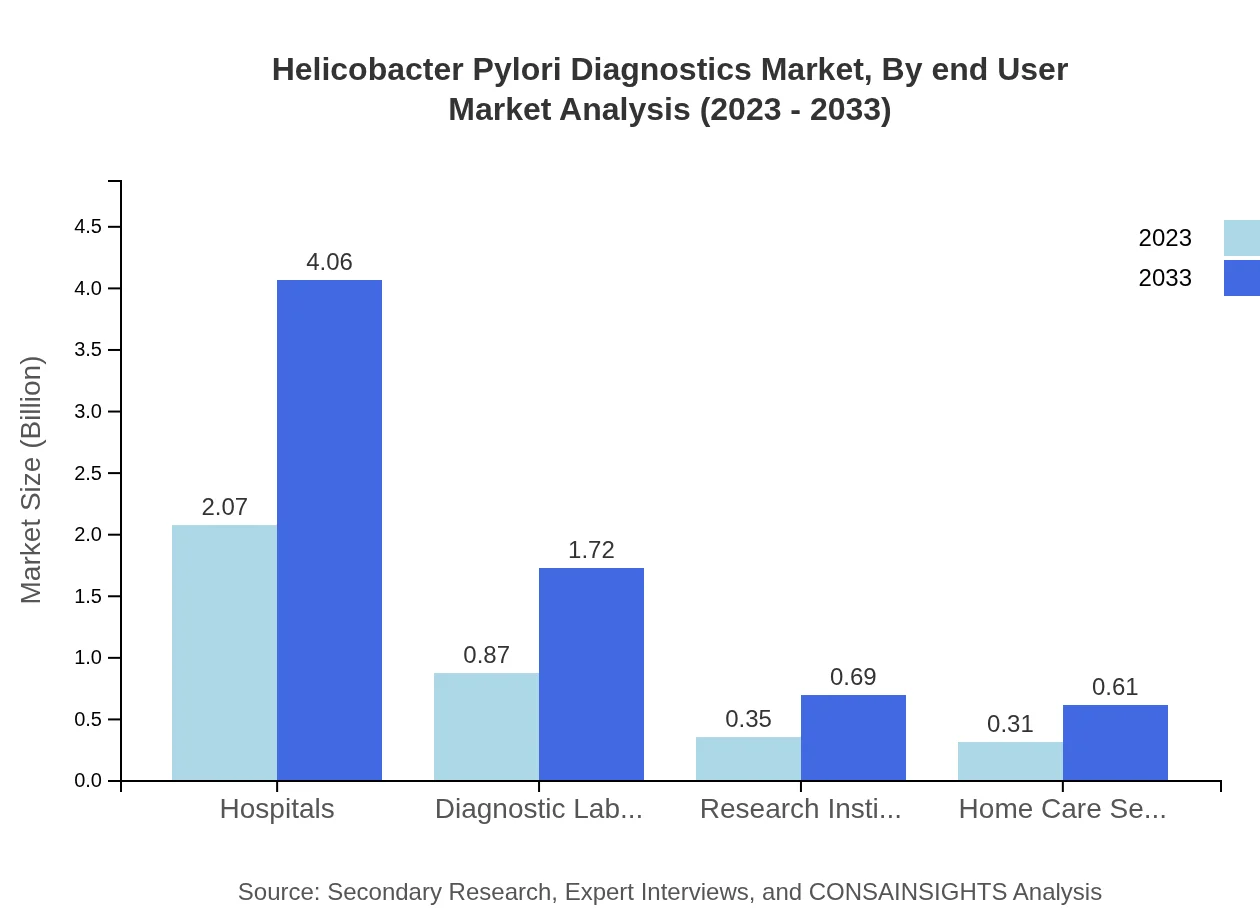
Helicobacter Pylori Diagnostics Market Analysis By End User
Hospitals are the primary end-users, capturing a market share of 57.4% in 2023. Diagnostic laboratories also hold a significant share, driven by their role in providing extensive diagnostic services. Home care settings and research institutes are emerging as promising areas for growth, maximizing patient reach.
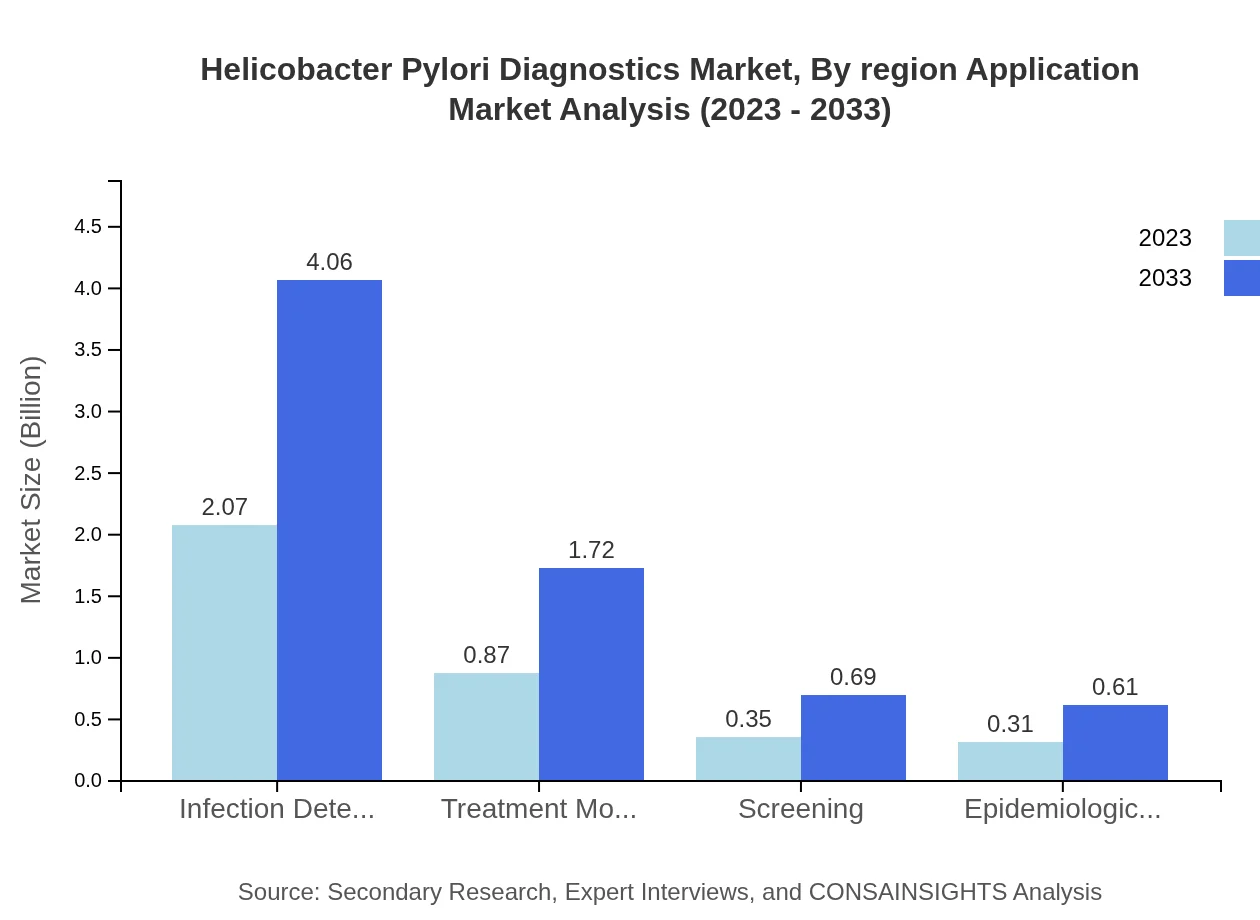
Helicobacter Pylori Diagnostics Market Analysis By Region Application
The primary applications of Helicobacter Pylori Diagnostics include infection detection, treatment monitoring, and screening for epidemiological studies. Each application shows a unique growth trajectory, with infection detection leading the demand due to rising awareness of gastrointestinal health.
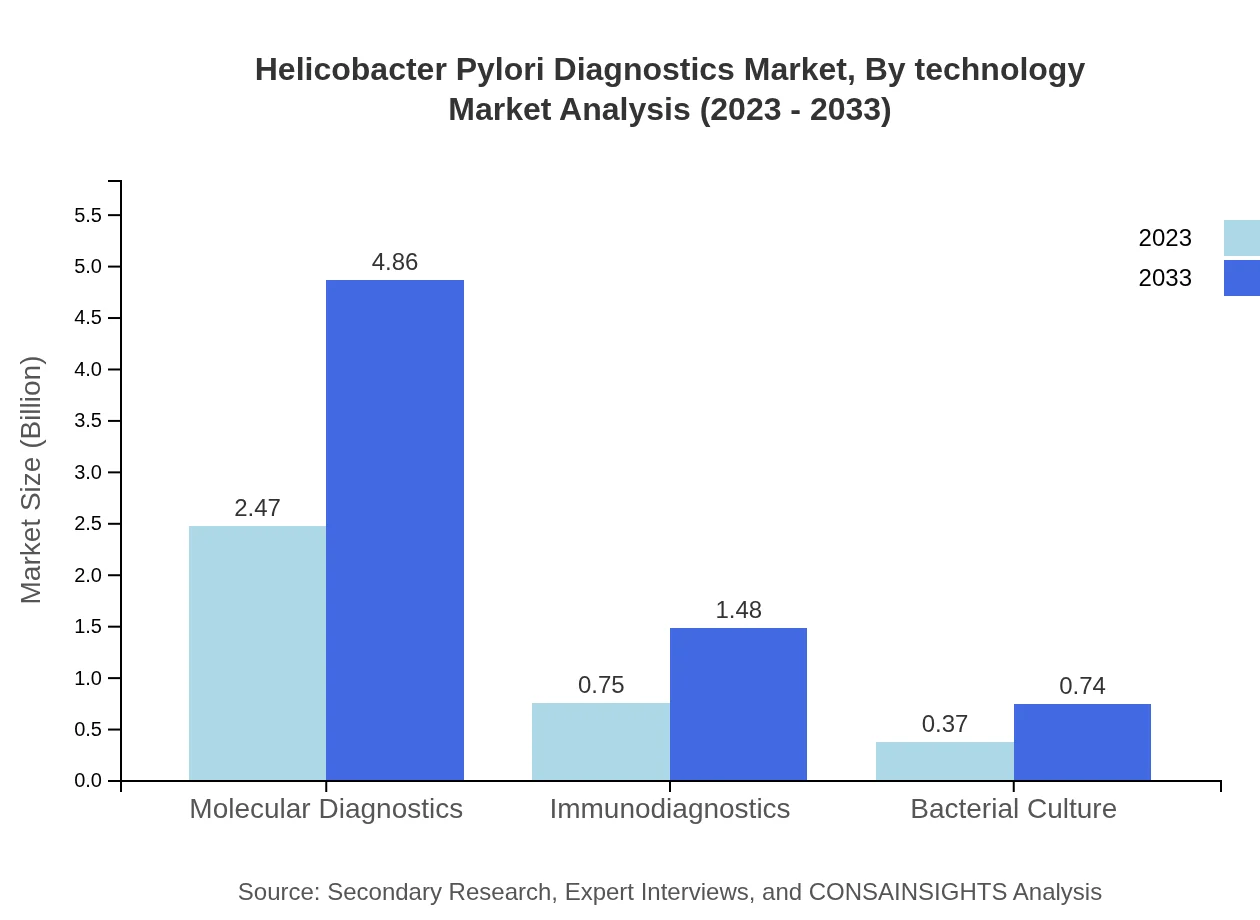
Helicobacter Pylori Diagnostics Market Analysis By Technology
Key technological advancements are shaping the Helicobacter Pylori Diagnostics sector. Molecular diagnostics dominate with a share of 68.63% in 2023, showcasing increased specificity and sensitivity, while immunodiagnostics and bacterial culture complement the range of diagnostic solutions.
Helicobacter Pylori Diagnostics Market Analysis By Region
Regionally, North America holds the largest share, continuously driven by technological innovation and healthcare access. Europe follows closely, bolstered by extensive healthcare networks. The growth in Asia-Pacific, although currently smaller, indicates a favorable trend as infrastructure improves and awareness increases.
Helicobacter Pylori Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Helicobacter Pylori Diagnostics Industry
Abbott Laboratories:
A leading player in the diagnostics sector with expansive portfolios in infectious disease testing, providing innovative solutions for detecting H. Pylori.Thermo Fisher Scientific:
Renowned for its robust diagnostic solutions, Thermo Fisher emphasizes molecular diagnostics and tests that facilitate effective H. Pylori detection.Roche Diagnostics:
A key contributor to diagnostic advancements, Roche offers a range of effective tests for Helicobacter Pylori, enhancing diagnostic pathways in healthcare.Danaher Corporation:
Through subsidiaries, Danaher focuses on innovative diagnostic technologies, expanding its influence in the Helicobacter Pylori diagnostics market.We're grateful to work with incredible clients.









FAQs
What is the market size of helicobacter Pylori Diagnostics?
The Helicobacter Pylori Diagnostics market size is projected to reach approximately $3.6 billion by 2033, growing at a CAGR of 6.8%. This growth reflects the increasing demand for accurate diagnostic tools in the healthcare sector.
What are the key market players or companies in this helicobacter Pylori Diagnostics industry?
Key players in the Helicobacter Pylori Diagnostics market include major companies that specialize in diagnostic solutions and medical devices, pivotal in driving innovation and expanding market reach through research and development initiatives.
What are the primary factors driving the growth in the helicobacter Pylori Diagnostics industry?
Growth drivers in the Helicobacter Pylori Diagnostics industry include rising global infection rates, enhanced healthcare infrastructure, increasing awareness of gastroenterological conditions, and technological advancements in diagnostic methods.
Which region is the fastest Growing in the helicobacter Pylori Diagnostics?
The fastest-growing region in the Helicobacter Pylori Diagnostics market is Europe, with the market expected to increase from $1.05 billion in 2023 to $2.06 billion by 2033, indicating robust demand and healthcare investment.
Does ConsaInsights provide customized market report data for the helicobacter Pylori Diagnostics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the Helicobacter Pylori Diagnostics industry, enabling businesses to make data-driven decisions based on unique market dynamics.
What deliverables can I expect from this helicobacter Pylori Diagnostics market research project?
Deliverables from the Helicobacter Pylori Diagnostics market research project typically include comprehensive market analysis reports, competitor profiles, future growth forecasts, and strategic recommendations for market entry or expansion.
What are the market trends of helicobacter Pylori Diagnostics?
Market trends in Helicobacter Pylori Diagnostics include the increasing use of molecular diagnostics, growth in home care testing solutions, and advancements in point-of-care testing technologies to enhance patient access and care quality.