Hemato Oncology Testing Market Report
Published Date: 31 January 2026 | Report Code: hemato-oncology-testing
Hemato Oncology Testing Market Size, Share, Industry Trends and Forecast to 2033
This report offers a detailed analysis of the Hemato Oncology Testing market from 2023 to 2033, including market size, growth rate, industry insights, and regional trends. It aims to provide valuable data for stakeholders and decision-makers in the healthcare sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
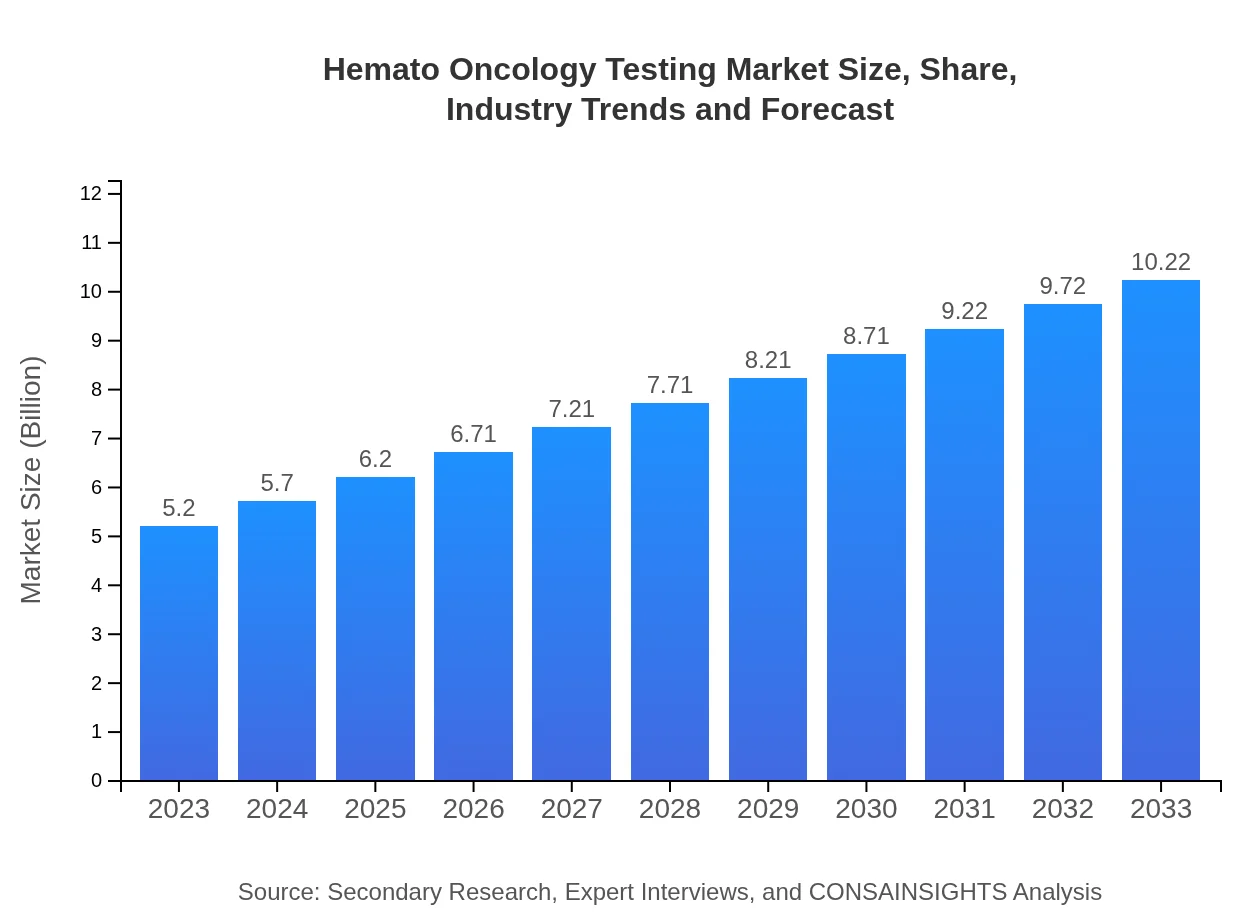
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Illumina, Inc., Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Hemato Oncology Testing Market Overview
Customize Hemato Oncology Testing Market Report market research report
- ✔ Get in-depth analysis of Hemato Oncology Testing market size, growth, and forecasts.
- ✔ Understand Hemato Oncology Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hemato Oncology Testing
What is the Market Size & CAGR of Hemato Oncology Testing market in 2023?
Hemato Oncology Testing Industry Analysis
Hemato Oncology Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hemato Oncology Testing Market Analysis Report by Region
Europe Hemato Oncology Testing Market Report:
Europe's market is anticipated to expand from $1.46 billion in 2023 to $2.86 billion by 2033. The growth is attributed to increasing incidences of blood cancers, an aging population, and stringent regulatory frameworks that promote product innovation.Asia Pacific Hemato Oncology Testing Market Report:
In the Asia Pacific region, the Hemato Oncology Testing market is expected to grow from $1.00 billion in 2023 to $1.97 billion by 2033. This growth is fueled by an increasing population at risk of hematological cancers, improved healthcare infrastructure, and rising awareness of advanced diagnostic techniques.North America Hemato Oncology Testing Market Report:
North America dominates the Hemato Oncology Testing market, projected to reach $3.54 billion by 2033 from $1.80 billion in 2023. This remarkable growth is primarily driven by advanced healthcare systems, significant R&D activities, and the presence of key market players.South America Hemato Oncology Testing Market Report:
The South American market is also poised for growth, with projections estimating an increase from $0.25 billion in 2023 to $0.48 billion by 2033. Factors such as rising healthcare investments and increased access to advanced testing methods are crucial for market expansion in this region.Middle East & Africa Hemato Oncology Testing Market Report:
The Middle East and Africa are projected to experience growth from $0.70 billion in 2023 to $1.37 billion by 2033. The market will benefit from increased access to healthcare services and rising investments in the healthcare sector, ensuring better diagnostic capabilities.Tell us your focus area and get a customized research report.
Hemato Oncology Testing Market Analysis By Test Type
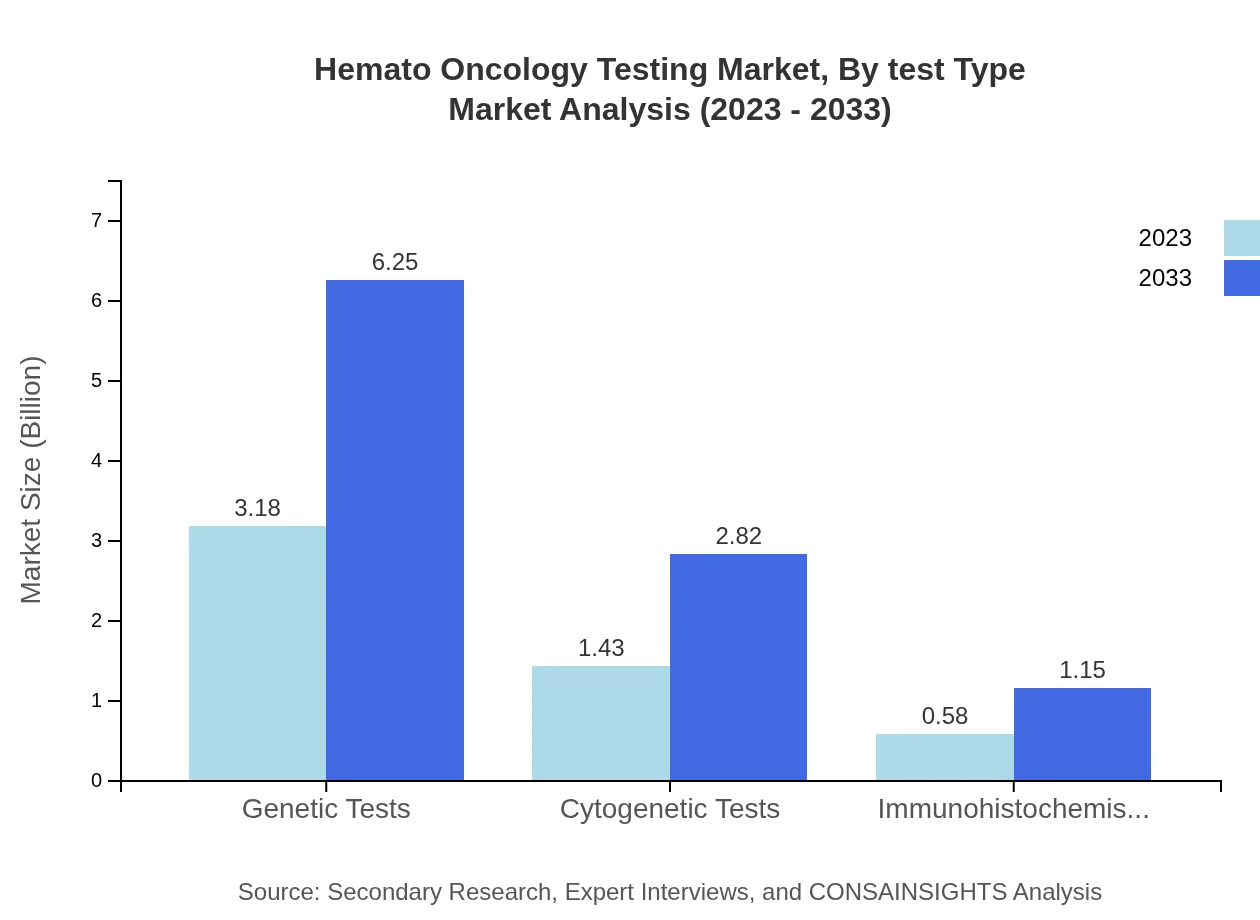
The Hemato-Oncology Testing Market is majorly segmented into genetic tests, molecular diagnostics, cytogenetic tests, and immunohistochemistry. Genetic tests dominate the market with over $3.18 billion in 2023, expected to rise to $6.25 billion by 2033, owing to their high specificity and ability to guide targeted therapies. Molecular diagnostics and cytogenetic tests also hold significant shares, providing essential insights into genetic variations associated with hematologic disorders.
Hemato Oncology Testing Market Analysis By Cancer Type
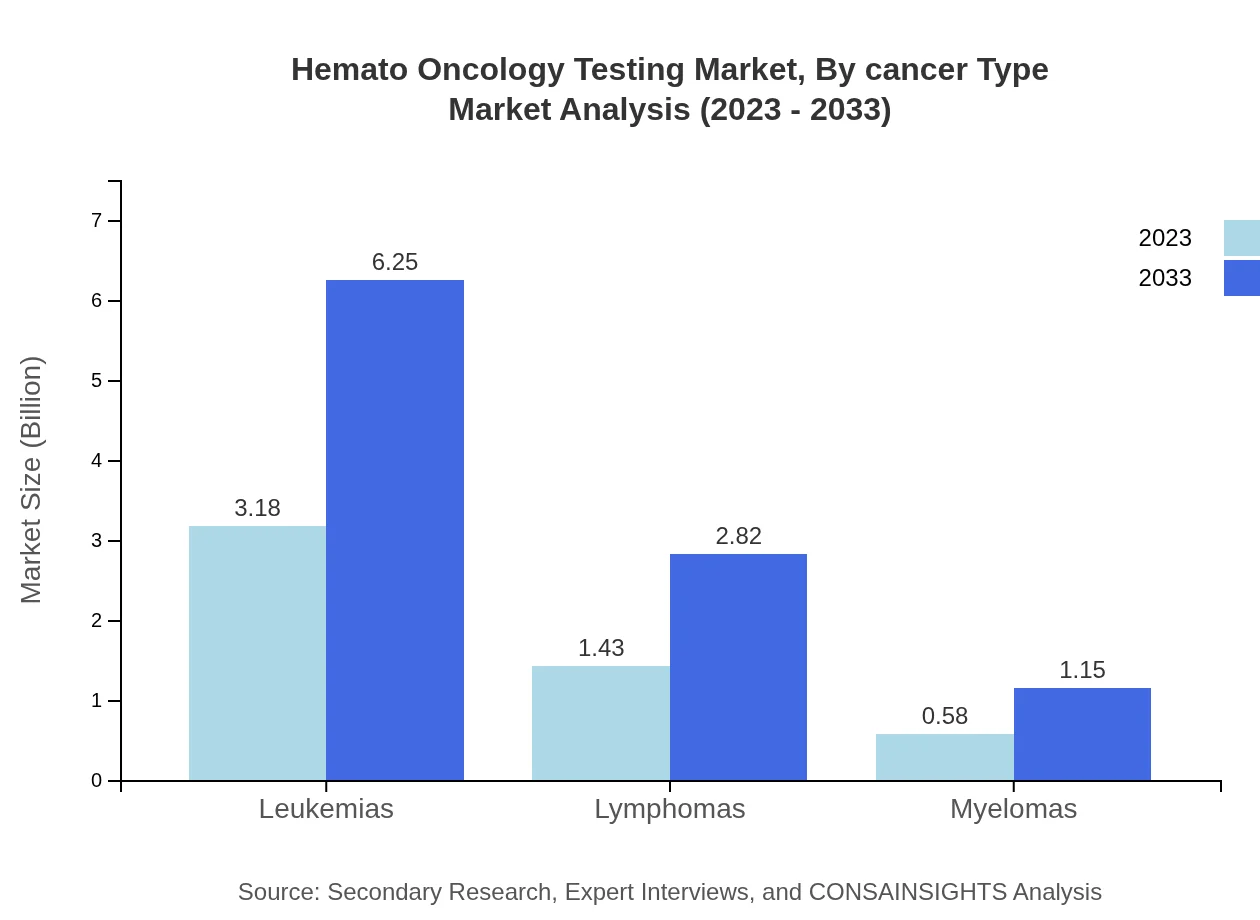
The market segmentation by cancer type primarily includes leukemias, lymphomas, and myelomas. Leukemias are the largest segment, boasting sales of $3.18 billion in 2023, projected to increase to $6.25 billion by 2033. Lymphomas and myelomas also present substantial growth potential, accounting for $1.43 billion and $0.58 billion in 2023, respectively.
Hemato Oncology Testing Market Analysis By Technology
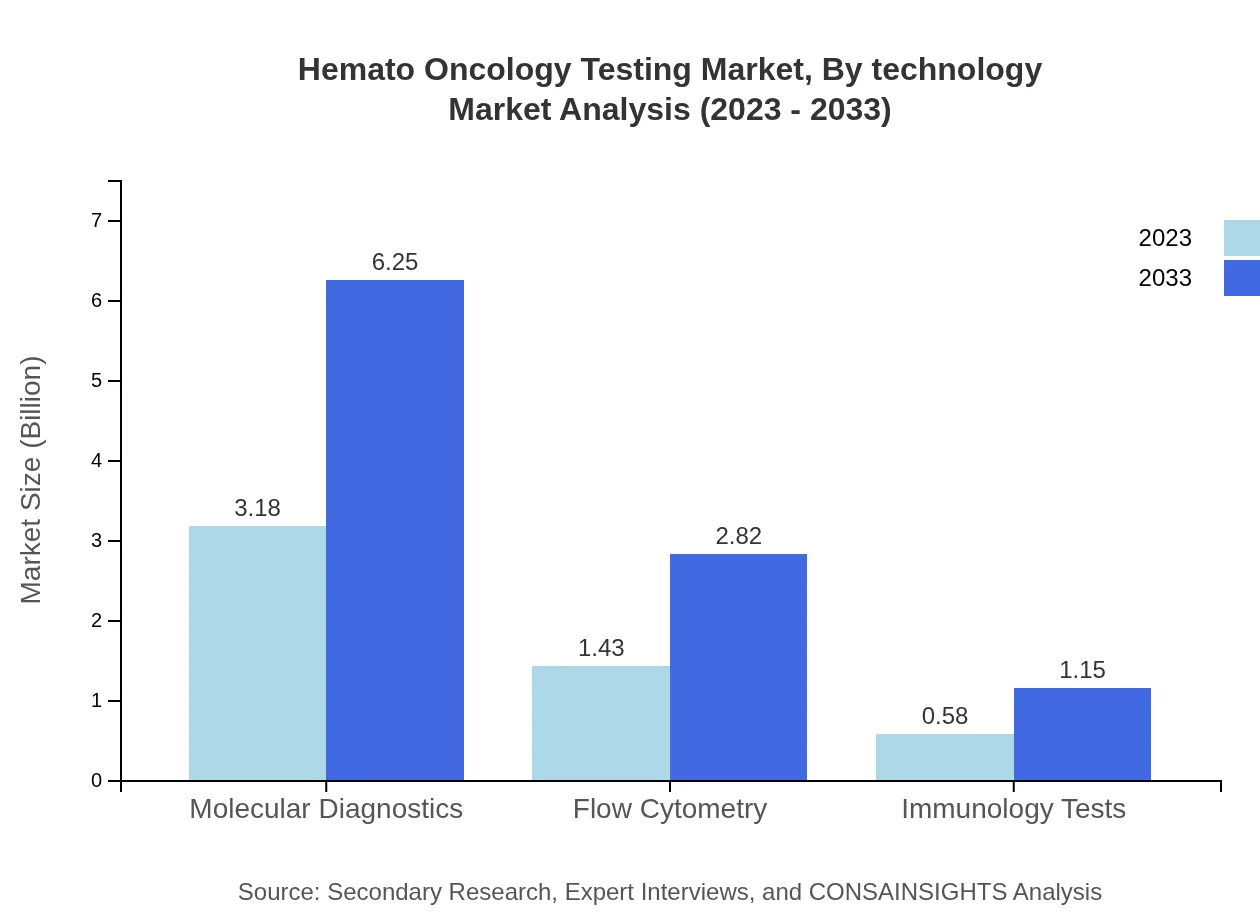
Technological advancements in Hemato Oncology Testing encompass flow cytometry, molecular diagnostics, and immunohistochemistry. Flow cytometry currently has a significant market presence, estimated at $1.43 billion in 2023 and anticipated to reach $2.82 billion by 2033. Advancements in molecular diagnostics are reshaping the market by enabling timely and precise detection of hematologic malignancies.
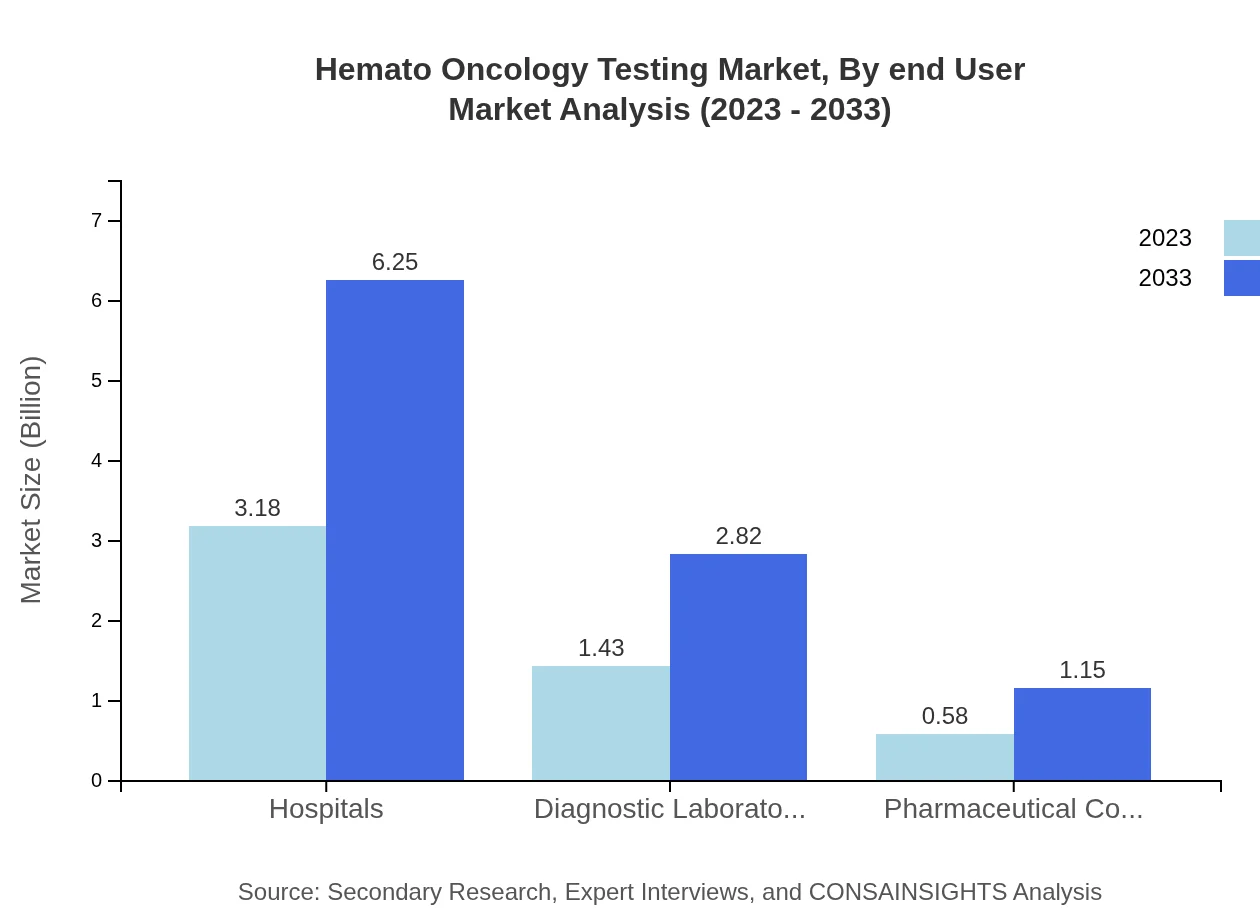
Hemato Oncology Testing Market Analysis By End User
The key end-users of Hemato Oncology Testing include hospitals, diagnostic laboratories, and pharmaceutical companies. Hospitals are the largest segment, accounting for a market size of $3.18 billion in 2023 and projected to maintain a steady growth trajectory. Diagnostic laboratories contribute significantly with an estimated value of $1.43 billion in 2023, driven by the high demand for accurate hematologic testing services.
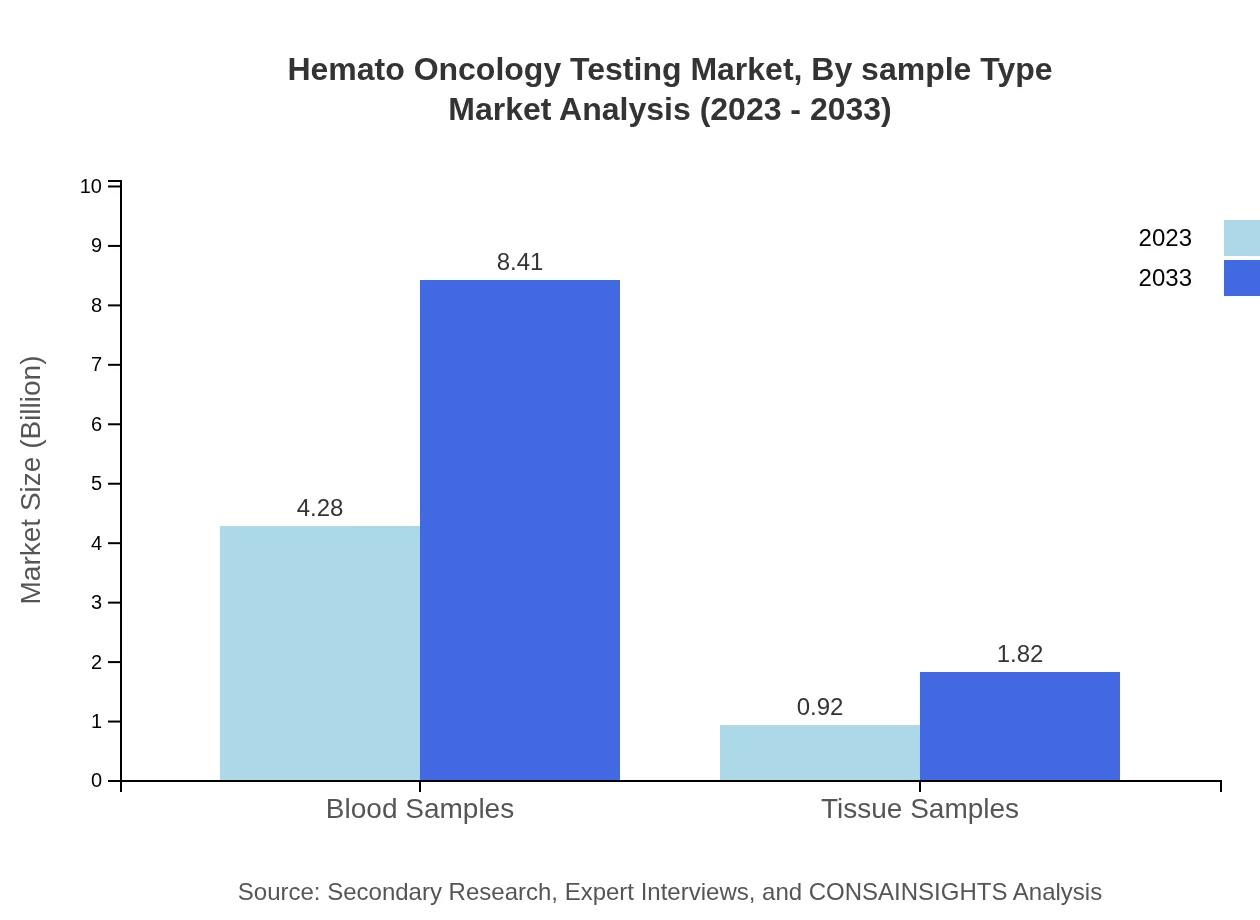
Hemato Oncology Testing Market Analysis By Sample Type
The analysis reveals a strong preference for blood samples in Hemato Oncology Testing, with sales reaching $4.28 billion in 2023. This trend is expected to continue as blood tests provide critical insights into hematologic conditions, with projected growth to $8.41 billion by 2033. Tissue samples are secondary, with current estimates at $0.92 billion, anticipated to reach $1.82 billion.
Hemato Oncology Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hemato Oncology Testing Industry
Roche Diagnostics:
Roche Diagnostics is a leader in the global diagnostics industry, offering a wide range of innovative solutions, including advanced hematology testing technologies that facilitate early diagnosis of blood cancers.Abbott Laboratories:
Abbott is committed to helping healthcare professionals provide accurate diagnoses with its cutting-edge technology in molecular diagnostics and genomic testing.Illumina, Inc.:
Illumina leads in genomic sequencing, providing the tools necessary for comprehensive hematological assessments and personalized treatment strategies.Thermo Fisher Scientific:
Thermo Fisher is renowned for its comprehensive range of laboratory products and services, particularly in molecular diagnostic testing for hematologic malignancies.We're grateful to work with incredible clients.









FAQs
What is the market size of hemato Oncology Testing?
The hemato-oncology testing market is valued at approximately $5.2 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.8%, suggesting robust growth driven by advancements in diagnostic technology and increased prevalence of blood cancers.
What are the key market players or companies in this hemato Oncology Testing industry?
Key players in the hemato-oncology testing industry include Siemens Healthineers, Thermo Fisher Scientific, Roche, Abbott Laboratories, and Quest Diagnostics, all of which contribute significantly to market growth through innovation and extensive product offerings.
What are the primary factors driving the growth in the hemato Oncology Testing industry?
Drivers for growth in hemato-oncology testing include rising incidences of hematological malignancies, advancements in molecular diagnostics, increased healthcare expenditure, and a shift toward personalized medicine, which enhances patient outcomes through targeted treatment strategies.
Which region is the fastest Growing in the hemato Oncology testing market?
North America is identified as the fastest-growing region in the hemato-oncology testing market, with market size expected to rise from $1.80 billion in 2023 to $3.54 billion by 2033, fueled by technological advancements and increasing healthcare investments.
Does ConsaInsights provide customized market report data for the hemato Oncology testing industry?
Yes, ConsaInsights offers customized market report data tailored to client specifications in the hemato-oncology testing industry, enabling stakeholders to gain insights specific to their interests and strategic needs.
What deliverables can I expect from this hemato Oncology testing market research project?
From this hemato-oncology testing market research project, clients can expect comprehensive reports detailing market size, growth forecasts, competitive landscape analysis, regional insights, and segment data that assist in strategic decision-making.
What are the market trends of hemato Oncology testing?
Current trends in hemato-oncology testing include increasing adoption of liquid biopsies, integration of artificial intelligence in diagnostics, rising demand for point-of-care testing, and growing prevalence of genetic testing to facilitate personalized therapy options.