Hemostasis Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: hemostasis-diagnostics
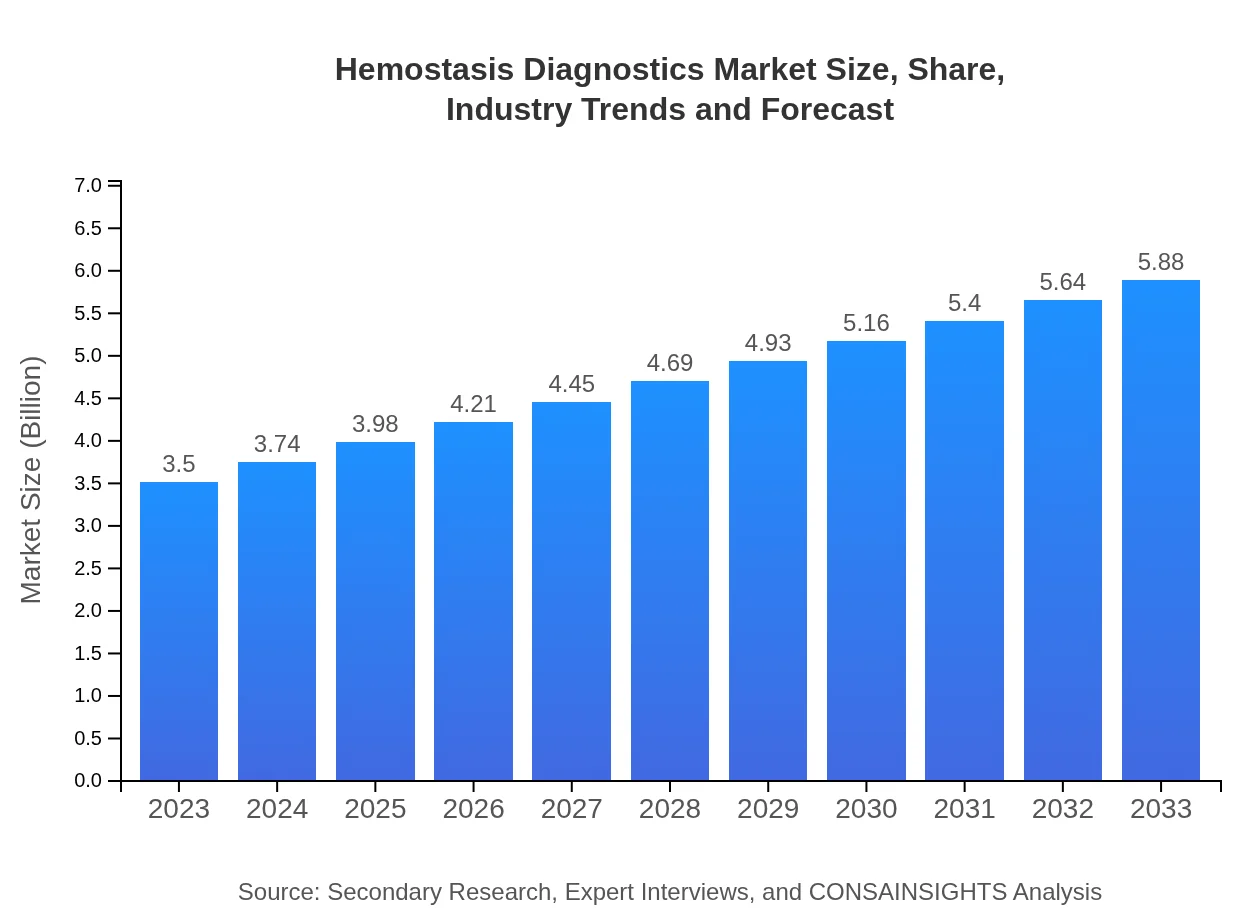
Hemostasis Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report covers a comprehensive analysis of the Hemostasis Diagnostics market from 2023 to 2033, providing insights into market size, growth factors, industry trends, key players, and regional analyses, alongside technological advancements shaping the sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 5.2% |
| 2033 Market Size | $5.88 Billion |
| Top Companies | Roche Diagnostics, Siemens Healthineers, Sysmex Corporation, Abbott Laboratories |
| Last Modified Date | 31 January 2026 |
Hemostasis Diagnostics Market Overview
Customize Hemostasis Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Hemostasis Diagnostics market size, growth, and forecasts.
- ✔ Understand Hemostasis Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hemostasis Diagnostics
What is the Market Size & CAGR of Hemostasis Diagnostics market in 2023?
Hemostasis Diagnostics Industry Analysis
Hemostasis Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hemostasis Diagnostics Market Analysis Report by Region
Europe Hemostasis Diagnostics Market Report:
In Europe, the market is set to increase from $0.96 billion in 2023 to approximately $1.61 billion by 2033. Factors such as regulatory support for medical devices and increased healthcare investments are bolstering market growth despite challenges posed by economic factors.Asia Pacific Hemostasis Diagnostics Market Report:
In the Asia Pacific region, the Hemostasis Diagnostics market is expected to grow from $0.70 billion in 2023 to approximately $1.17 billion by 2033. This growth is attributed to increasing healthcare expenditure, rising prevalence of thrombotic disorders, and expanding patient awareness regarding early diagnosis and treatment options.North America Hemostasis Diagnostics Market Report:
The North American market remains the largest, expected to grow from $1.15 billion in 2023 to $1.94 billion in 2033. This growth is driven by advanced healthcare systems, high awareness about blood-related diseases, and robust spending on diagnostic technologies.South America Hemostasis Diagnostics Market Report:
South America is witnessing gradual growth in the Hemostasis Diagnostics market, projected to increase from $0.33 billion in 2023 to $0.56 billion by 2033. Factors such as improving healthcare infrastructure and increasing investments in medical technologies contribute to this growth.Middle East & Africa Hemostasis Diagnostics Market Report:
The Middle East and Africa market is expected to grow from $0.35 billion in 2023 to $0.59 billion by 2033, supported primarily by rising awareness about diagnostic testing and improvements in healthcare infrastructure.Tell us your focus area and get a customized research report.
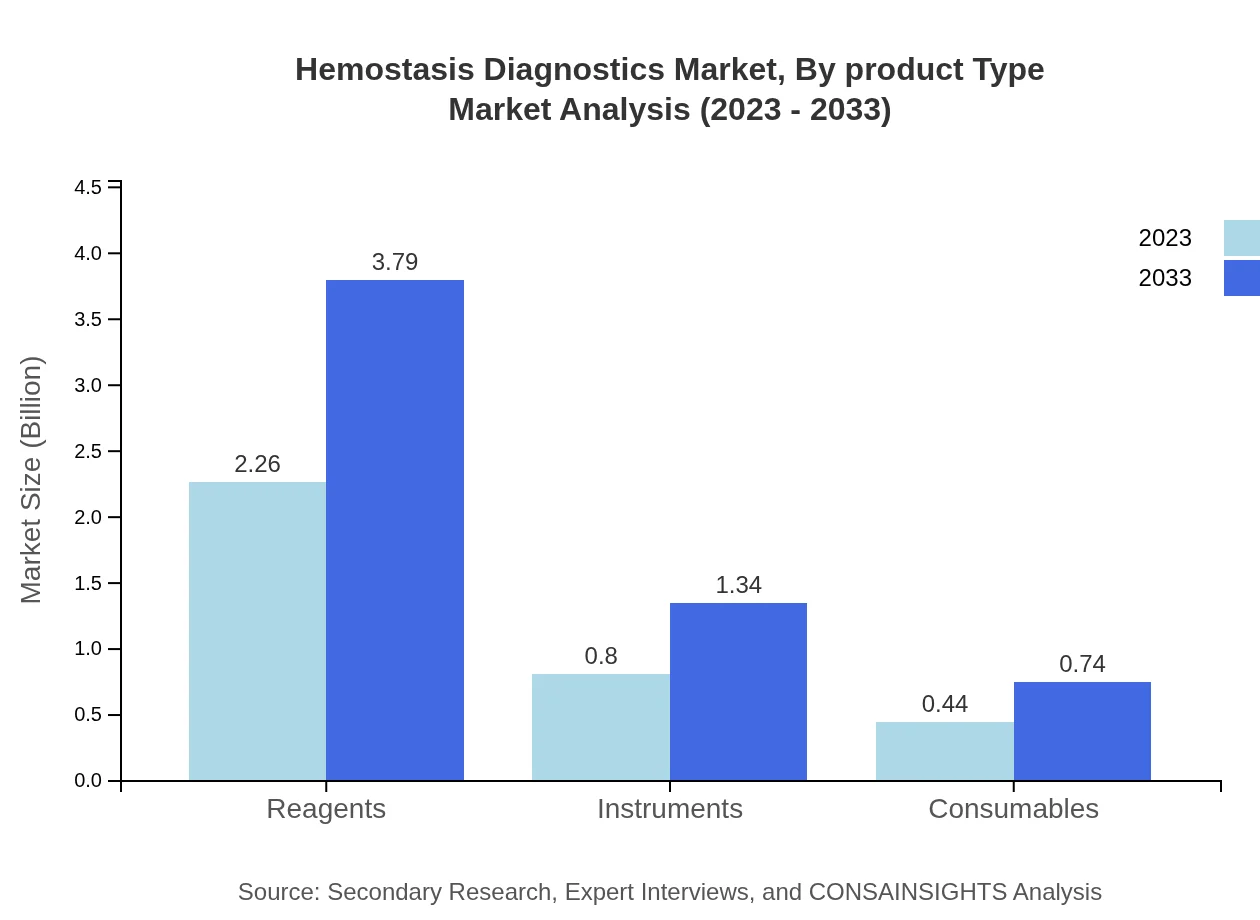
Hemostasis Diagnostics Market Analysis By Product Type
In 2023, the Hemostasis Diagnostics market by product type shows reagents leading with a size of $2.26 billion, expected to rise to $3.79 billion by 2033. Instruments and consumables follow, with respective sizes of $0.80 billion and $0.44 billion in 2023, growing to $1.34 billion and $0.74 billion by 2033.
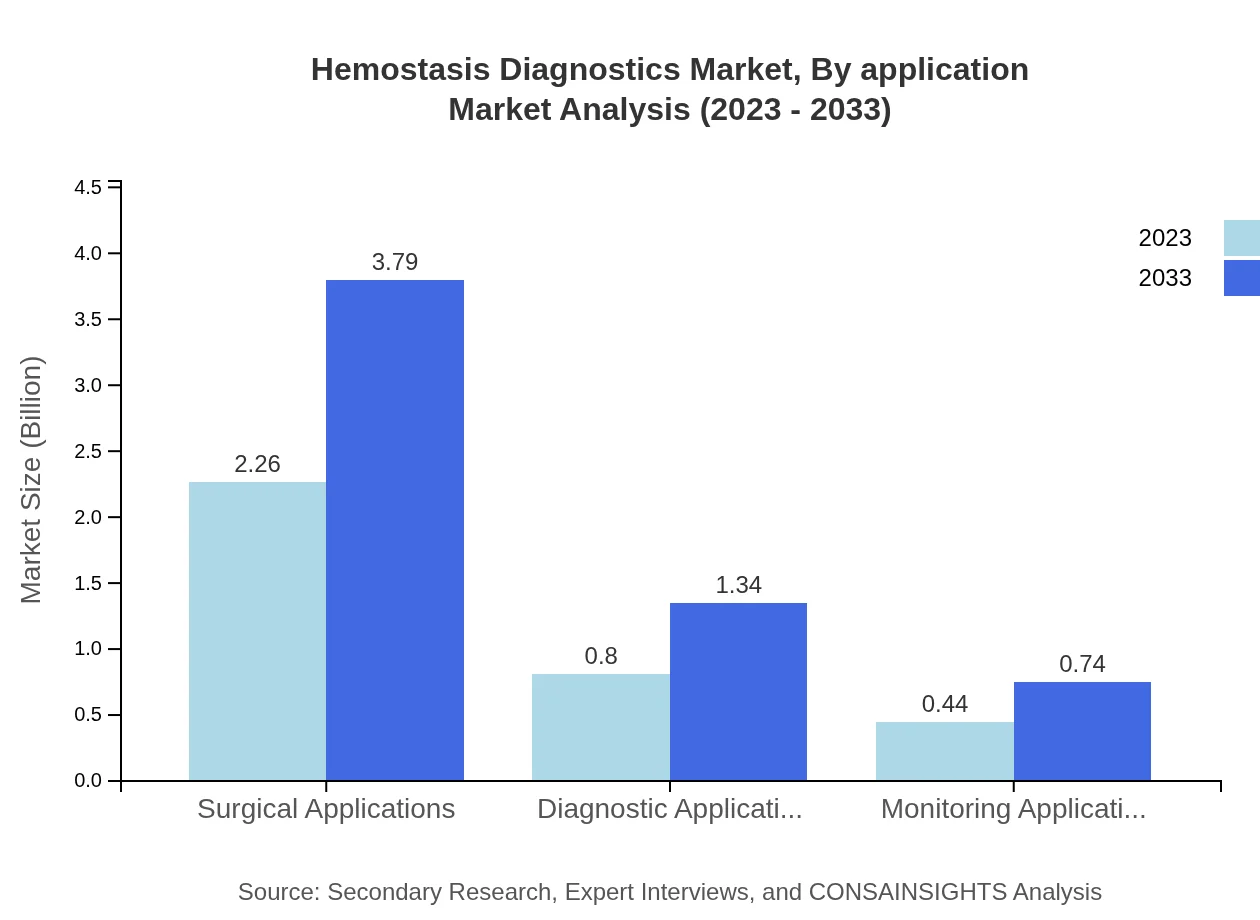
Hemostasis Diagnostics Market Analysis By Application
Surgical applications are the most significant segment with a market size of $2.26 billion in 2023, projected to reach $3.79 billion by 2033. Diagnostic applications are anticipated to grow from $0.80 billion to $1.34 billion, with monitoring applications increasing from $0.44 billion to $0.74 billion during the same period.
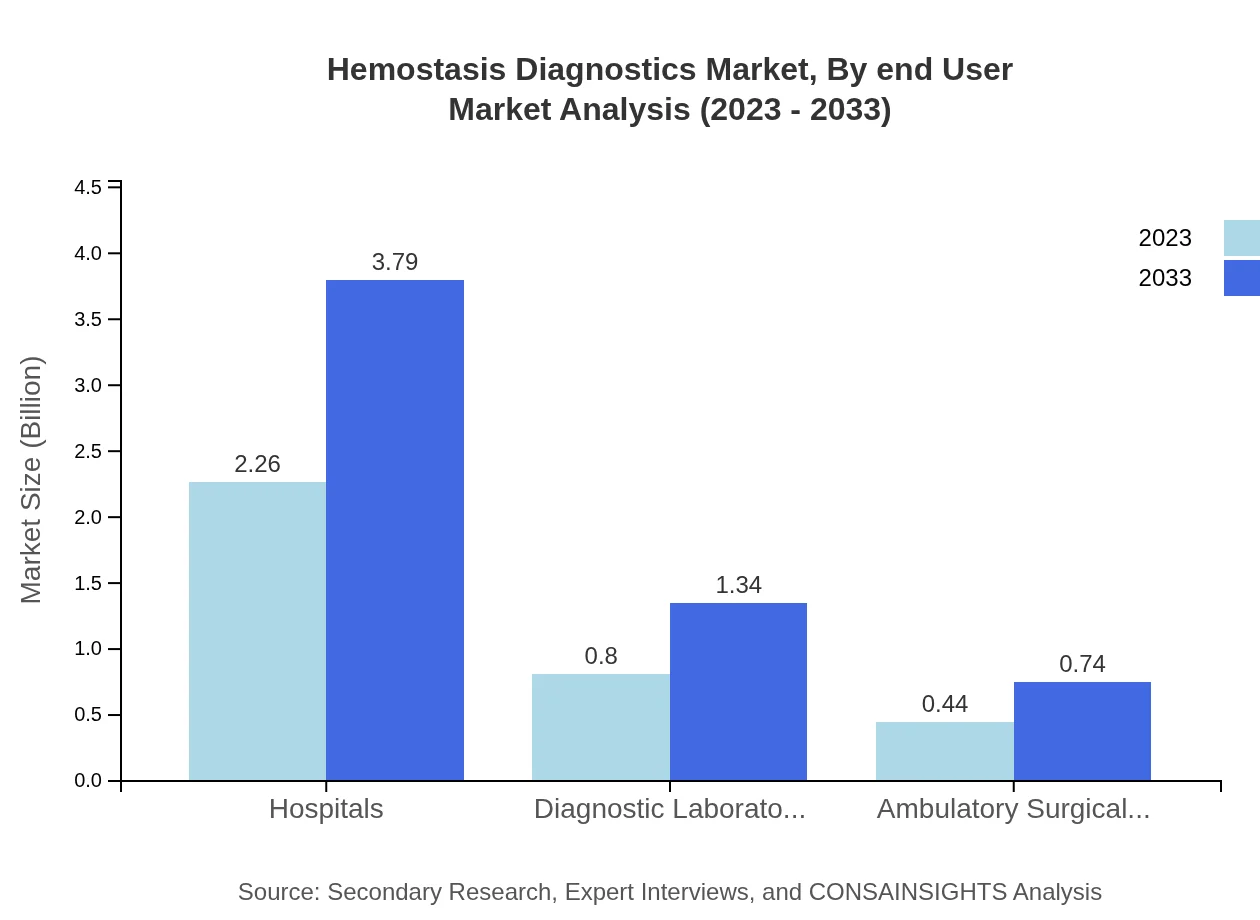
Hemostasis Diagnostics Market Analysis By End User
Hospitals dominate the end-user segment, representing 64.56% of the market share in 2023, maintaining a similar share in 2033. Diagnostic laboratories and ambulatory surgical centers account for 22.85% and 12.59% respectively, with sizes of $0.80 billion and $0.44 billion in 2023, both anticipated to grow by 2033.
Hemostasis Diagnostics Market Analysis By Region Substitution
The regional segmentation shows North America leading in penetration and market activity, with Europe and Asia Pacific closely following. Companies are strategically focusing on emerging markets in Asia and South America due to increasing healthcare investments and rising awareness.
Hemostasis Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hemostasis Diagnostics Industry
Roche Diagnostics:
A leading player in the diagnostic market with a strong portfolio of hemostasis products and innovations in testing technologies.Siemens Healthineers:
Known for its advanced diagnostics solutions and laboratory instruments, Siemens continues to innovate in the Hemostasis Diagnostics space.Sysmex Corporation:
Offers a wide range of hemostasis testing instruments and reagents, contributing significantly to advancements in laboratory efficiency.Abbott Laboratories:
Provides various diagnostic testing solutions, with a particular focus on expanding its hemostasis product lineup.We're grateful to work with incredible clients.









FAQs
What is the market size of hemostasis Diagnostics?
The Hemostasis Diagnostics market is valued at approximately $3.5 billion in 2023, with an anticipated compound annual growth rate (CAGR) of 5.2%, projecting significant growth over the next decade. By 2033, this market is expected to grow substantially, driven by advancements in technology and increasing healthcare demands.
What are the key market players or companies in this hemostasis Diagnostics industry?
Key players in the Hemostasis Diagnostics industry include established companies such as Siemens Healthineers, Roche, Abbott Laboratories, BioMérieux, and Instrumentation Laboratory. These organizations are pivotal in driving innovation and expanding their product lines to meet growing market needs.
What are the primary factors driving the growth in the hemostasis Diagnostics industry?
Factors driving growth in the Hemostasis Diagnostics industry include a rising prevalence of blood disorders, technological advancements in diagnostic equipment, increasing demand for accurate and timely diagnosis, and growth in healthcare expenditures across various regions, enhancing healthcare access.
Which region is the fastest Growing in the hemostasis Diagnostics?
The Asia-Pacific region is the fastest-growing area in the Hemostasis Diagnostics market, with a projected market increase from $0.70 billion in 2023 to $1.17 billion by 2033. This growth is fueled by increasing healthcare investments and rising awareness of blood-related disorders.
Does ConsaInsights provide customized market report data for the hemostasis Diagnostics industry?
Yes, ConsaInsights offers customized market report data for the Hemostasis Diagnostics industry, catering to specific client needs. This tailored approach ensures that clients receive the most relevant and actionable insights for their business strategies and market challenges.
What deliverables can I expect from this hemostasis Diagnostics market research project?
Deliverables from the Hemostasis Diagnostics market research project typically include detailed market analysis reports, trend forecasts, competitive landscape assessments, and insights into customer preferences. Clients can expect actionable recommendations to guide strategic decisions.
What are the market trends of hemostasis Diagnostics?
Current market trends in Hemostasis Diagnostics indicate significant technological advancements in automated diagnostic devices, increased integration of digital solutions, and a shift towards point-of-care testing. These trends enhance the efficiency and accuracy of hemostasis testing in clinical environments.