Hepatitis Diagnostic Test Market Report
Published Date: 31 January 2026 | Report Code: hepatitis-diagnostic-test
Hepatitis Diagnostic Test Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Hepatitis Diagnostic Test market, detailing key insights, trends, and projections from 2023 to 2033, alongside market segmentation, regional analysis, and key players impacting this essential healthcare sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
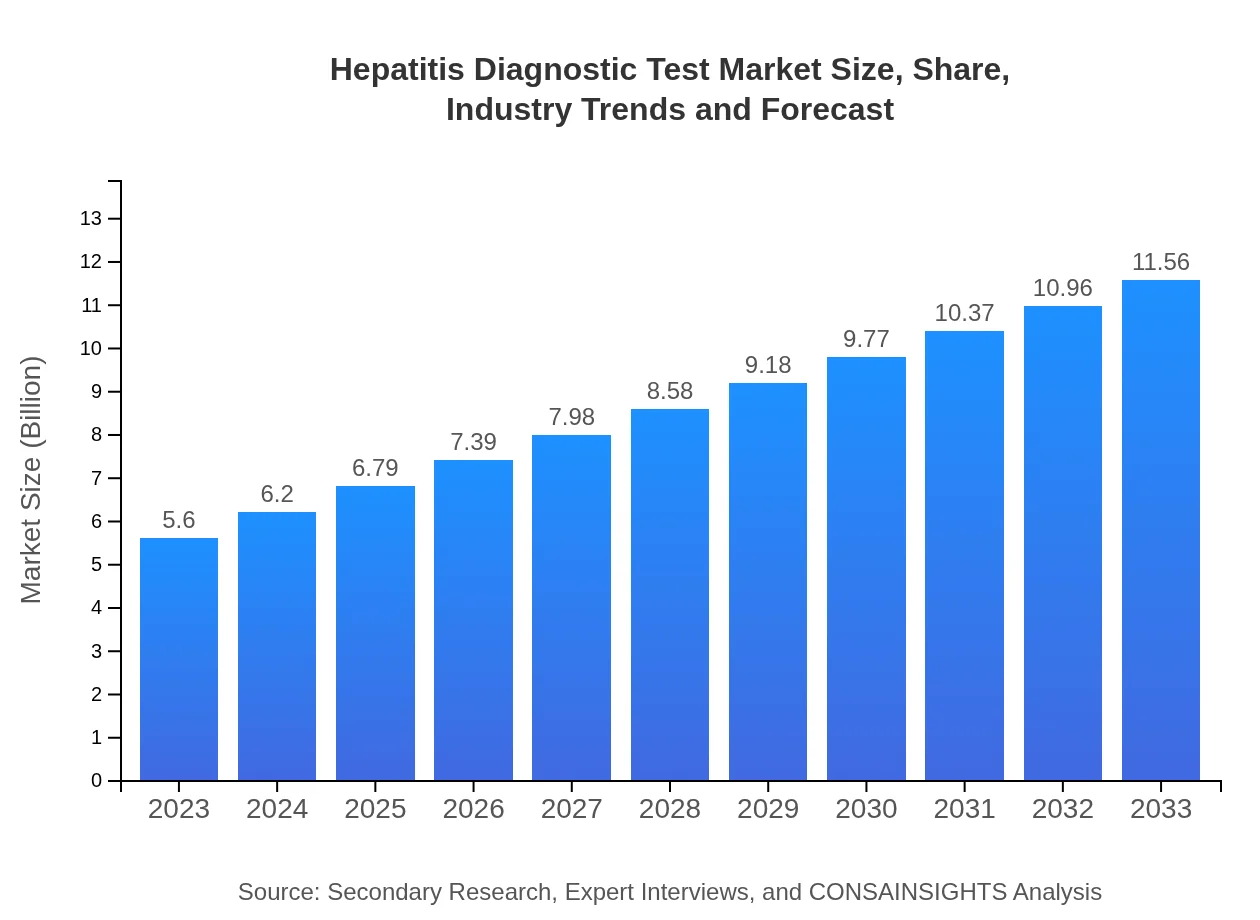
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.3% |
| 2033 Market Size | $11.56 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Quest Diagnostics, Bio-Rad Laboratories, Ortho Clinical Diagnostics |
| Last Modified Date | 31 January 2026 |
Hepatitis Diagnostic Test Market Overview
Customize Hepatitis Diagnostic Test Market Report market research report
- ✔ Get in-depth analysis of Hepatitis Diagnostic Test market size, growth, and forecasts.
- ✔ Understand Hepatitis Diagnostic Test's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hepatitis Diagnostic Test
What is the Market Size & CAGR of Hepatitis Diagnostic Test market in 2023?
Hepatitis Diagnostic Test Industry Analysis
Hepatitis Diagnostic Test Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hepatitis Diagnostic Test Market Analysis Report by Region
Europe Hepatitis Diagnostic Test Market Report:
Europe's market is anticipated to expand from $1.99 billion in 2023 to $4.10 billion by 2033. The growth can be attributed to heightened awareness and adoption of screening programs aimed at identifying hepatitis cases early on, as well as rigorous government regulations supporting diagnostic accuracy.Asia Pacific Hepatitis Diagnostic Test Market Report:
In the Asia Pacific region, the Hepatitis Diagnostic Test market is projected to grow from $0.95 billion in 2023 to $1.97 billion by 2033, driven by increasing healthcare expenditure and rising awareness about hepatitis infections. Collaborations between government entities and private health organizations bolster testing initiatives, further expanding market growth.North America Hepatitis Diagnostic Test Market Report:
North America shows significant potential in the Hepatitis Diagnostic Test market, with the market size projected to rise from $1.86 billion in 2023 to $3.83 billion by 2033. The increase is driven by advanced healthcare systems, widespread availability of diagnostic tests, and strong focus on preventive healthcare initiatives.South America Hepatitis Diagnostic Test Market Report:
The South American Hepatitis Diagnostic Test market is expected to grow from $0.30 billion in 2023 to $0.62 billion by 2033. This growth is fueled by improving healthcare infrastructure and government initiatives dedicated to hepatitis prevention and management, strengthening community health outreach programs.Middle East & Africa Hepatitis Diagnostic Test Market Report:
The Hepatitis Diagnostic Test market in the Middle East and Africa is projected to increase from $0.50 billion in 2023 to approximately $1.03 billion by 2033. Factors contributing to this growth include improving healthcare facilities and initiatives directed towards disease prevention and awareness campaigns in the region.Tell us your focus area and get a customized research report.
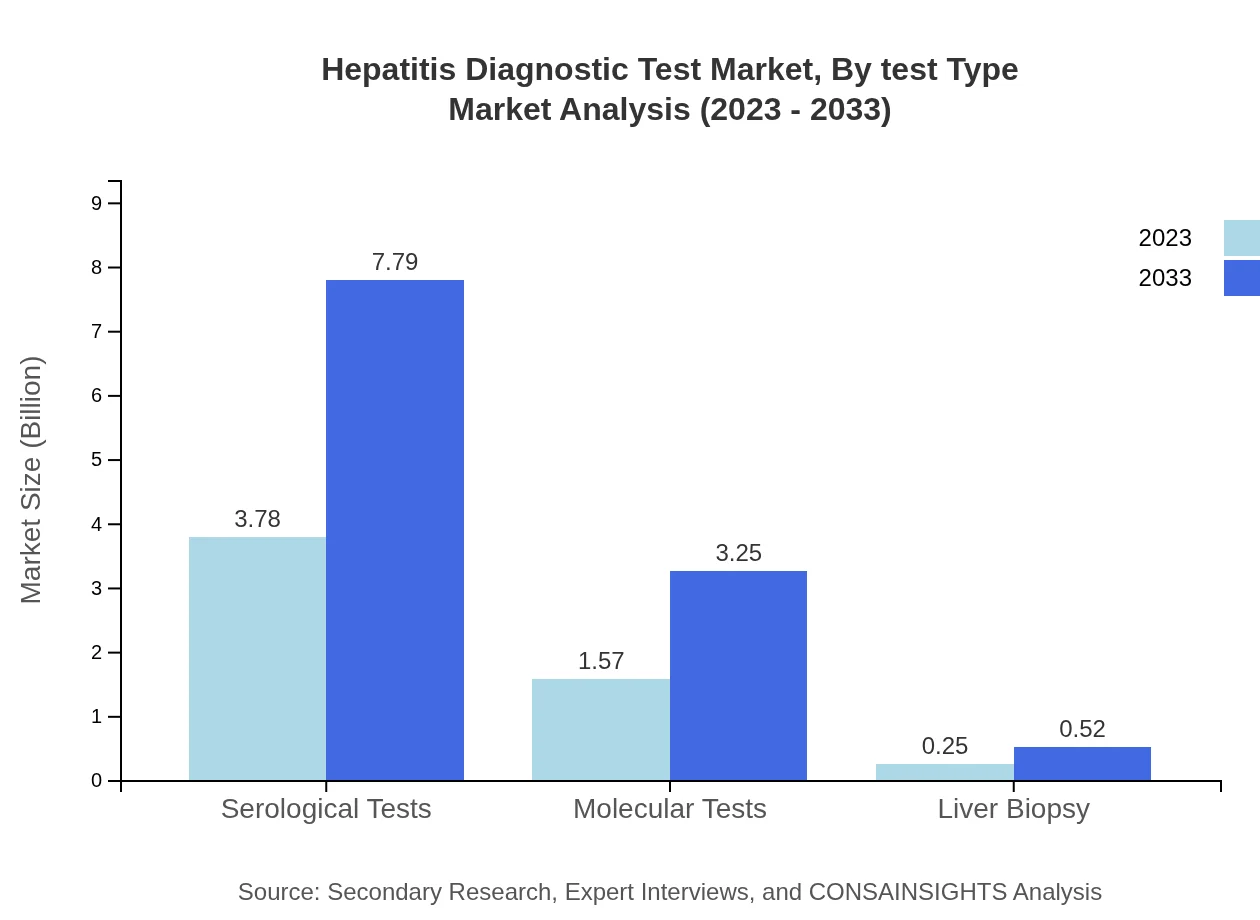
Hepatitis Diagnostic Test Market Analysis By Test Type
The Hepatitis Diagnostic Test market, by test type, includes Serological Tests, which represent a significant share of 67.42% in 2023, while revenue is expected to grow from $3.78 billion to $7.79 billion by 2033. Molecular Tests hold a share of 28.08%, projected to expand from $1.57 billion to $3.25 billion over the same period. The relative contributions of less common methods such as liver biopsy (4.5%) also showcase the diverse testing approaches in use.
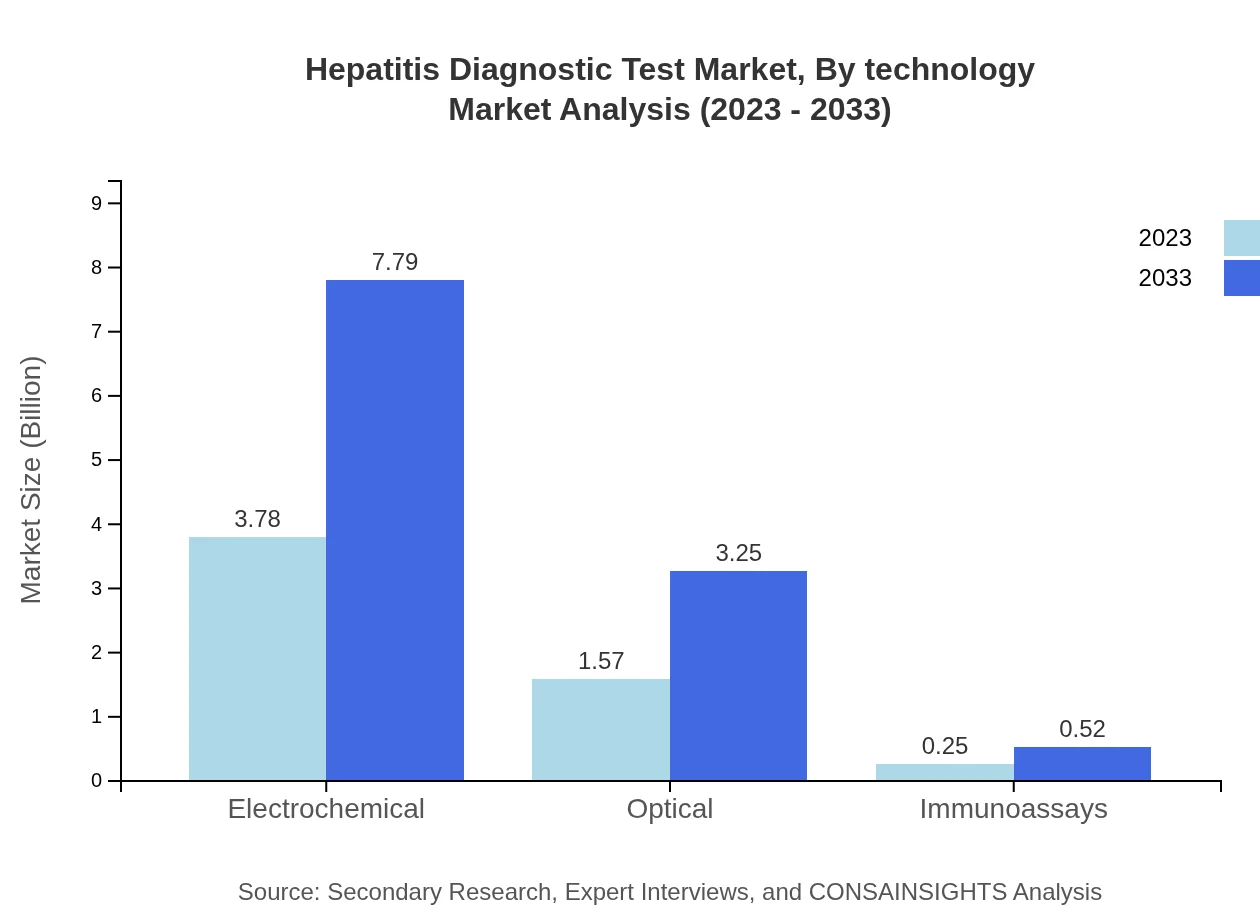
Hepatitis Diagnostic Test Market Analysis By Technology
In terms of technology, the market is dominated by Electrochemical Assays (67.42% share), with expected revenue growth from $3.78 billion to $7.79 billion from 2023 to 2033. Other technologies, including Optical Methods (28.08% share) and Immunoassays (4.5%), illustrate the varied landscape of technical innovation within the Hepatitis Diagnostic Test market.
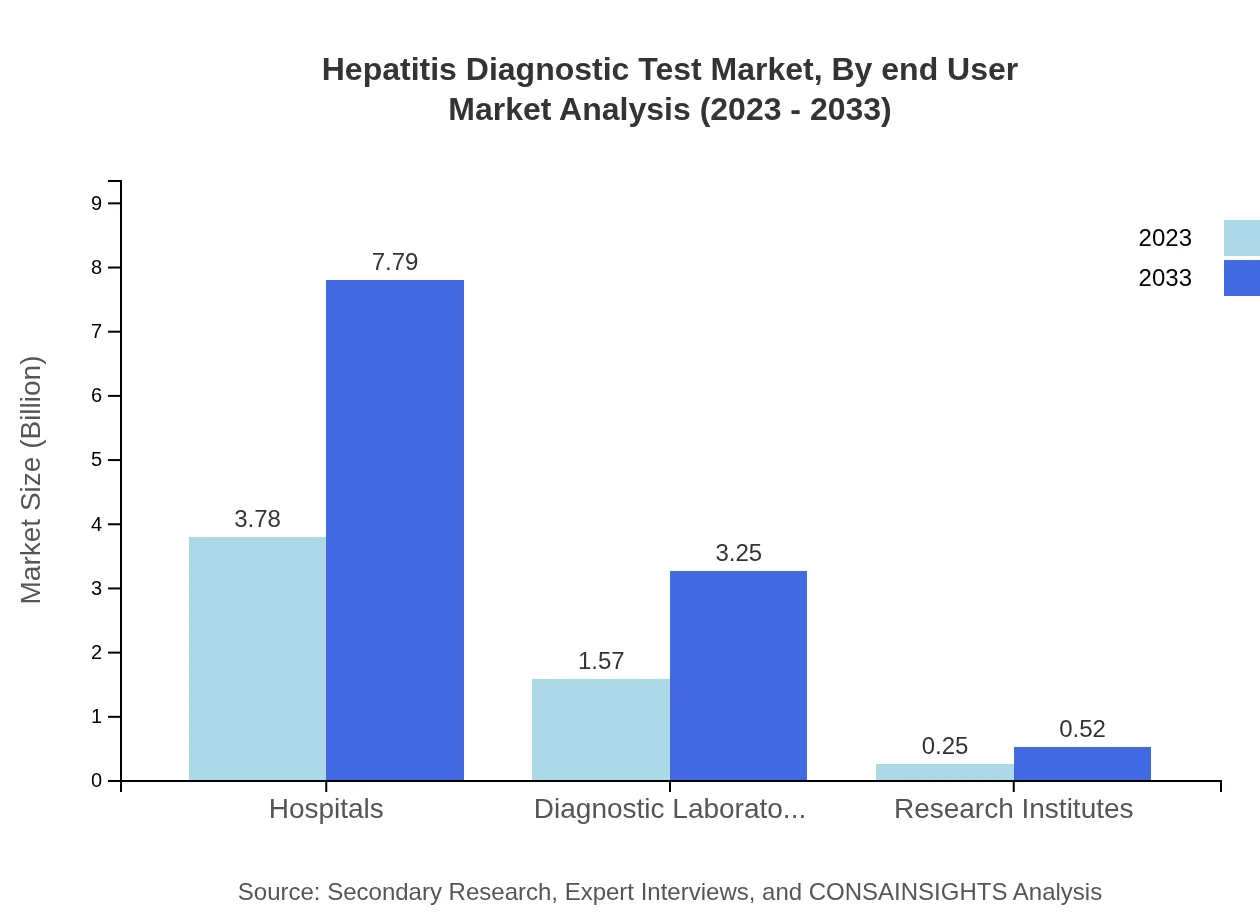
Hepatitis Diagnostic Test Market Analysis By End User
The Hepatitis Diagnostic Test market predominantly serves hospitals, holding a substantial portion of the market due to their capacity for comprehensive patient care. Revenue from diagnostic laboratories is also notable, indicating a growing trend in specialized testing services. Research Institutes contribute with a focus on innovative testing techniques and public health research.
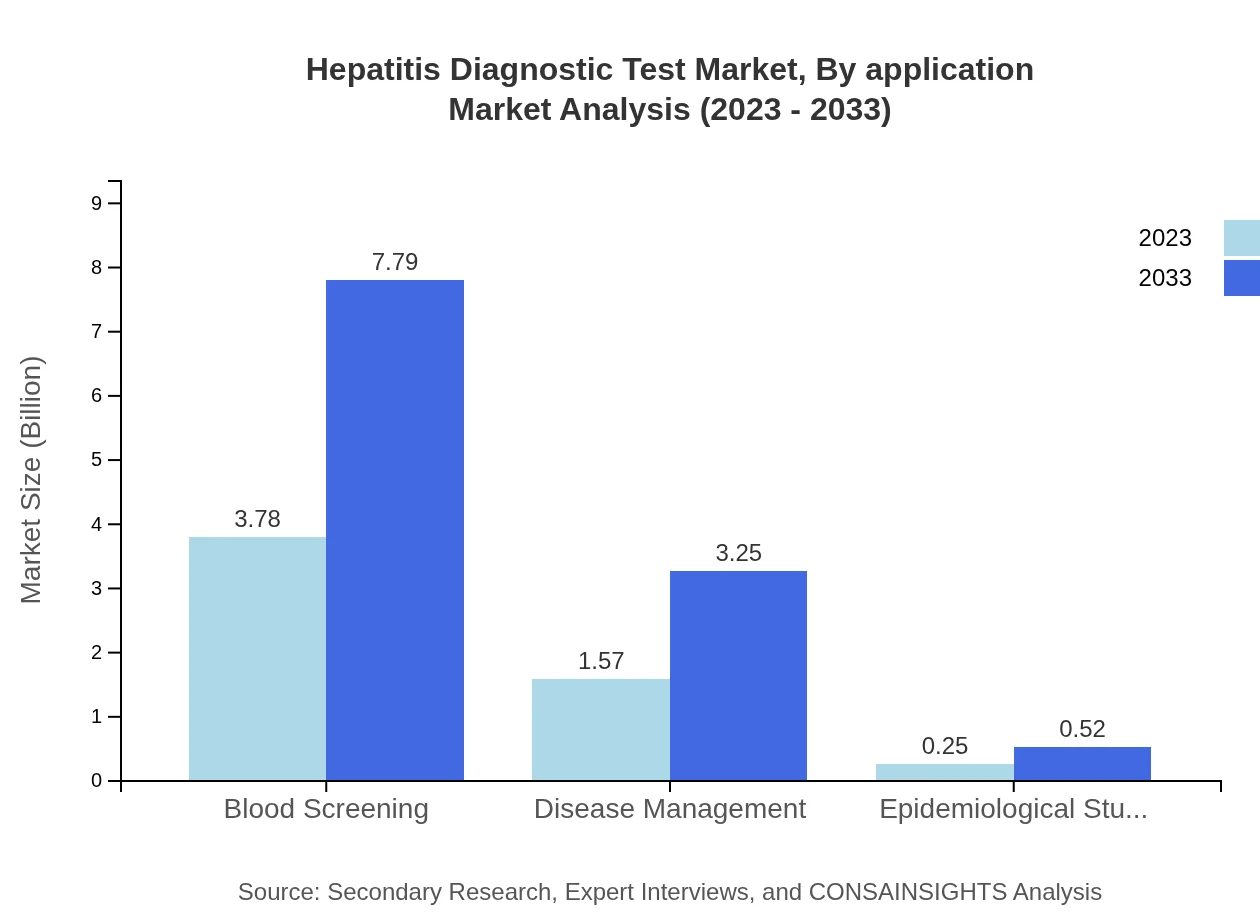
Hepatitis Diagnostic Test Market Analysis By Application
Key applications of Hepatitis Diagnostic Tests include Blood Screening, Disease Management, and Epidemiological Studies. Blood screening remains the largest segment, underpinning the need for extensive public health efforts to identify and control hepatitis outbreaks, while disease management strategies evolve towards more personalized healthcare solutions.
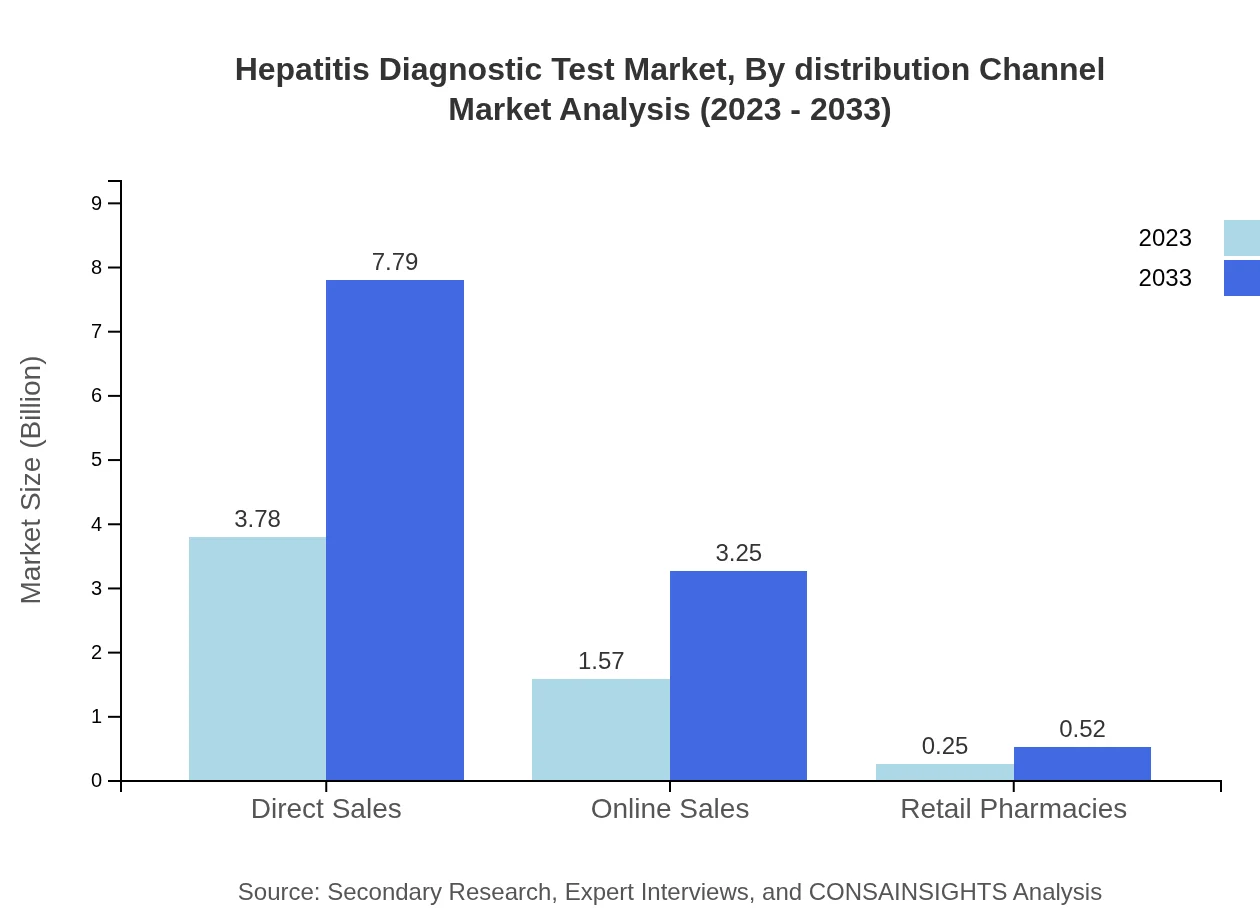
Hepatitis Diagnostic Test Market Analysis By Distribution Channel
Distribution channels for Hepatitis Diagnostic Tests include Direct Sales, which account for the significant majority of the market, and Online Sales, which are increasingly gaining traction. The shift towards e-commerce reflects consumer preferences and technological advancements in healthcare distribution.
Hepatitis Diagnostic Test Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hepatitis Diagnostic Test Industry
Roche Diagnostics:
Roche Diagnostics is a leader in the field of diagnostics, offering a range of tests and platforms for hepatitis diagnosis. Their innovative products are well-regarded for accuracy and reliability.Abbott Laboratories:
Abbott Laboratories provides a diverse portfolio of diagnostic solutions, including hepatitis tests designed to aid in patient management and public health initiatives.Quest Diagnostics:
Quest Diagnostics specializes in laboratory testing and health information technology. They play a key role in providing accurate testing and have fostered partnerships with various healthcare facilities.Bio-Rad Laboratories:
Bio-Rad Laboratories is known for its advances in immunology and molecular diagnostics, contributing significantly to hepatitis testing technology.Ortho Clinical Diagnostics:
Ortho Clinical Diagnostics focuses on providing innovative testing solutions for both infectious diseases and routine diagnostics, including hepatitis.We're grateful to work with incredible clients.









FAQs
What is the market size of hepatitis Diagnostic Test?
The hepatitis diagnostic test market is currently valued at approximately $5.6 billion, with a compound annual growth rate (CAGR) of 7.3%. This growth indicates a rising demand for Hepatitis diagnostic solutions over the coming years.
What are the key market players or companies in this hepatitis Diagnostic Test industry?
Key players in the hepatitis diagnostic test market include established healthcare companies specializing in diagnostic solutions and technology innovations. Notable firms contribute to research and development, enhancing diagnostic capabilities across various regions.
What are the primary factors driving the growth in the hepatitis Diagnostic Test industry?
The growth in the hepatitis diagnostic test market is fueled by increasing prevalence rates, advancing technology in molecular diagnostics, and rising awareness about hepatitis management and screening across populations.
Which region is the fastest Growing in the hepatitis Diagnostic Test?
The fastest-growing region in the hepatitis diagnostic test market is Europe, projected to expand from $1.99 billion in 2023 to $4.10 billion by 2033, showcasing a strong demand for diagnostic technologies in healthcare.
Does ConsaInsights provide customized market report data for the hepatitis Diagnostic Test industry?
Yes, ConsaInsights offers tailored market reports that provide in-depth analysis and customized data to meet specific business needs within the hepatitis diagnostic test industry, ensuring comprehensive insights.
What deliverables can I expect from this hepatitis Diagnostic Test market research project?
Deliverables include detailed market analysis, regional insights, segmentation data, key player profiles, and trend forecasts, providing an extensive overview of the hepatitis diagnostic test market for informed decision-making.
What are the market trends of hepatitis Diagnostic Test?
Current trends in the hepatitis diagnostic test market include the emergence of advanced molecular testing techniques, increased focus on automation in laboratories, and a shift towards rapid point-of-care testing solutions.