Hepatitis Test Solution Diagnosis Market Report
Published Date: 31 January 2026 | Report Code: hepatitis-test-solution-diagnosis
Hepatitis Test Solution Diagnosis Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Hepatitis Test Solution Diagnosis market, covering key trends, regional insights, market size forecasts from 2023 to 2033, and an overview of the competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
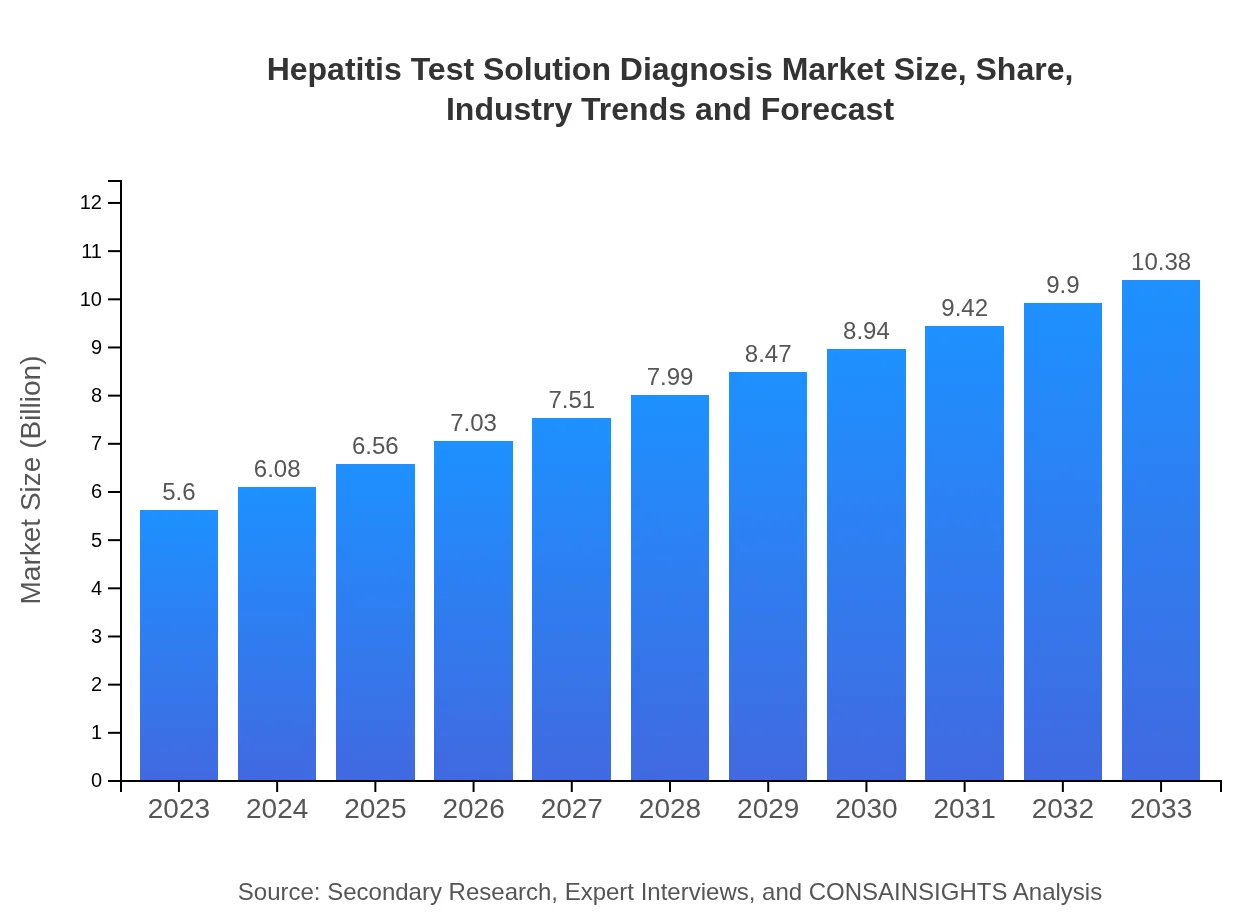
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $10.38 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, bioMérieux |
| Last Modified Date | 31 January 2026 |
Hepatitis Test Solution Diagnosis Market Overview
Customize Hepatitis Test Solution Diagnosis Market Report market research report
- ✔ Get in-depth analysis of Hepatitis Test Solution Diagnosis market size, growth, and forecasts.
- ✔ Understand Hepatitis Test Solution Diagnosis's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hepatitis Test Solution Diagnosis
What is the Market Size & CAGR of Hepatitis Test Solution Diagnosis market in 2023 and 2033?
Hepatitis Test Solution Diagnosis Industry Analysis
Hepatitis Test Solution Diagnosis Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hepatitis Test Solution Diagnosis Market Analysis Report by Region
Europe Hepatitis Test Solution Diagnosis Market Report:
With a current market value of about $1.47 billion in 2023, the European Hepatitis Test Solution Diagnosis market is projected to nearly double, reaching $2.73 billion by 2033. The growth can be attributed to strict government regulations promoting early screening and rising public health awareness.Asia Pacific Hepatitis Test Solution Diagnosis Market Report:
In the Asia Pacific region, the Hepatitis Test Solution Diagnosis market was valued at approximately $1.12 billion in 2023 and is projected to grow to $2.08 billion by 2033. The increasing prevalence of Hepatitis infections, coupled with improvements in healthcare infrastructure, drives this growth. Countries like China and India are crucial markets due to their large populations and rising disease awareness.North America Hepatitis Test Solution Diagnosis Market Report:
The North American market for Hepatitis Test Solution Diagnosis is substantial, valued at approximately $1.82 billion in 2023 and expected to grow to $3.37 billion by 2033. The region is marked by advanced healthcare systems and high diagnostic rates, supported by robust R&D activities.South America Hepatitis Test Solution Diagnosis Market Report:
South America is witnessing significant growth in the Hepatitis Test Solution Diagnosis market, which was valued at around $0.52 billion in 2023 and is anticipated to reach $0.96 billion by 2033. Efforts to increase screening programs and improve access to healthcare services are driving this upward trend.Middle East & Africa Hepatitis Test Solution Diagnosis Market Report:
In the Middle East and Africa, the Hepatitis Test Solution Diagnosis market had a size of $0.67 billion in 2023, anticipated to expand to $1.24 billion by 2033. This growth reflects an increasing focus on infectious diseases and initiatives to improve testing accessibility.Tell us your focus area and get a customized research report.
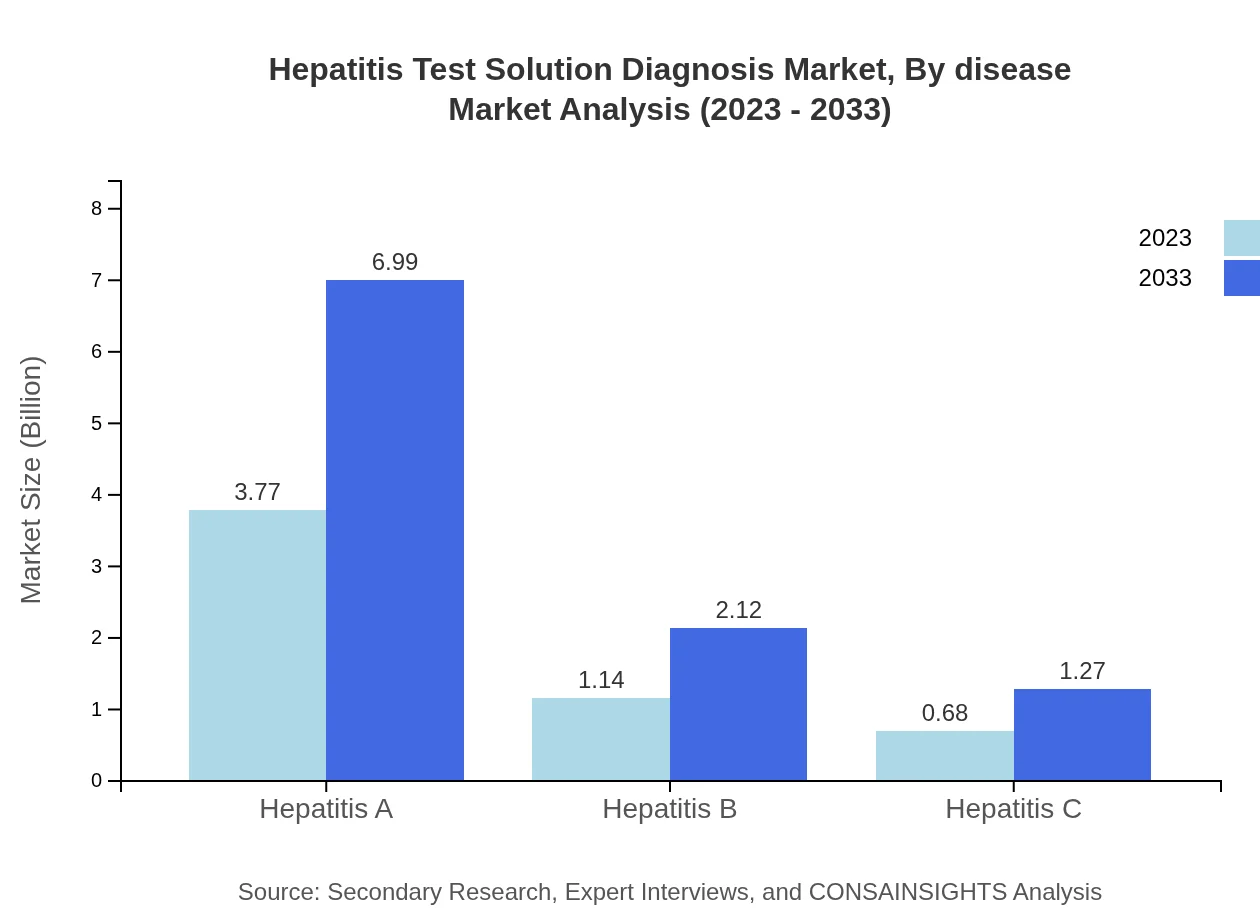
Hepatitis Test Solution Diagnosis Market Analysis By Disease
The market by disease is predominantly led by Hepatitis A, with a market size of $3.77 billion in 2023 and projected to grow to $6.99 billion by 2033, maintaining a share of 67.38%. Hepatitis B follows, initially valued at $1.14 billion in 2023 and expected to reach $2.12 billion by 2033. Lastly, Hepatitis C demonstrates growth from $0.68 billion in 2023 to $1.27 billion by 2033.
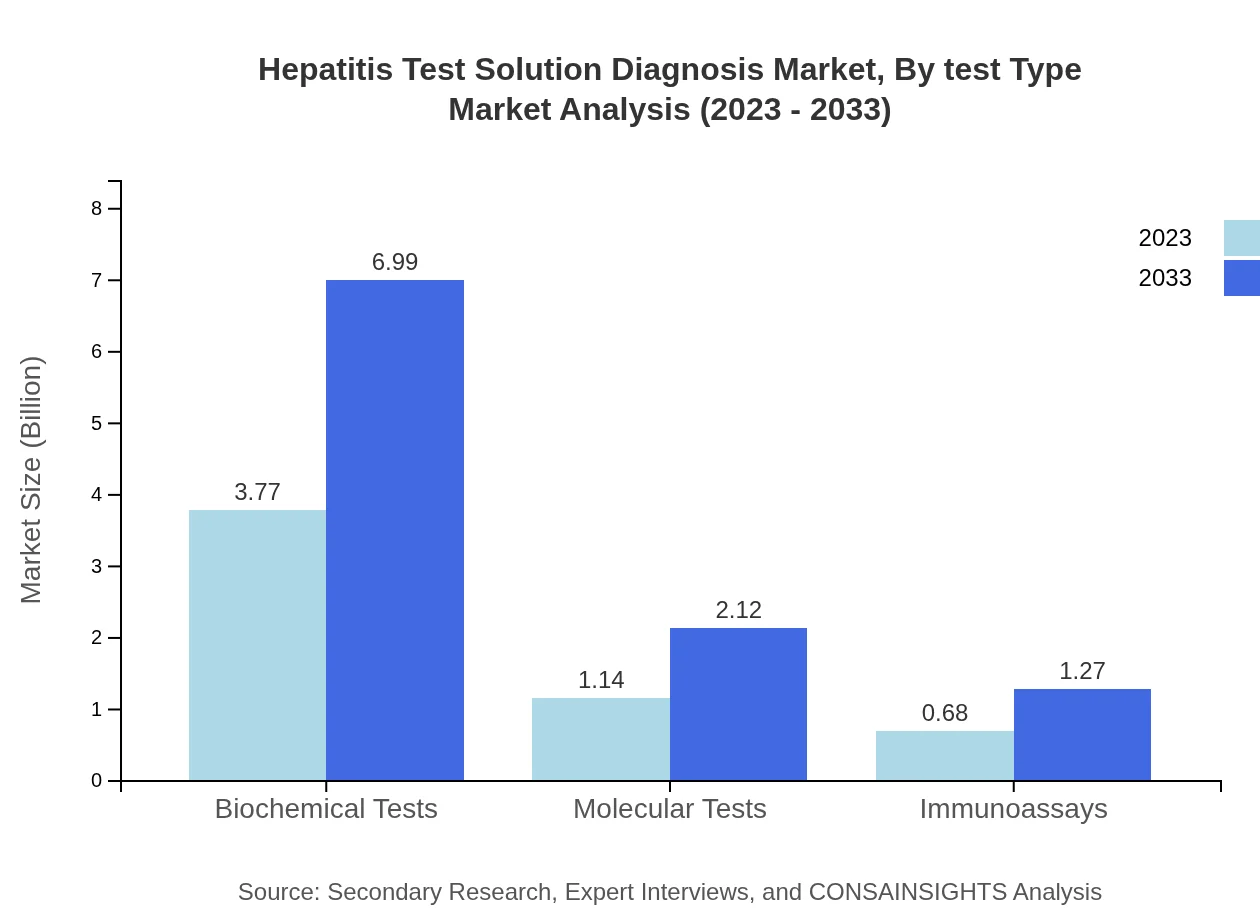
Hepatitis Test Solution Diagnosis Market Analysis By Test Type
In terms of test types, the Hepatitis Test Solution market is primarily composed of biochemical tests (67.38% market share), projected to grow from $3.77 billion in 2023 to $6.99 billion by 2033. Molecular tests, although smaller in share, will increase in importance, expanding from $1.14 billion to $2.12 billion during the same period.
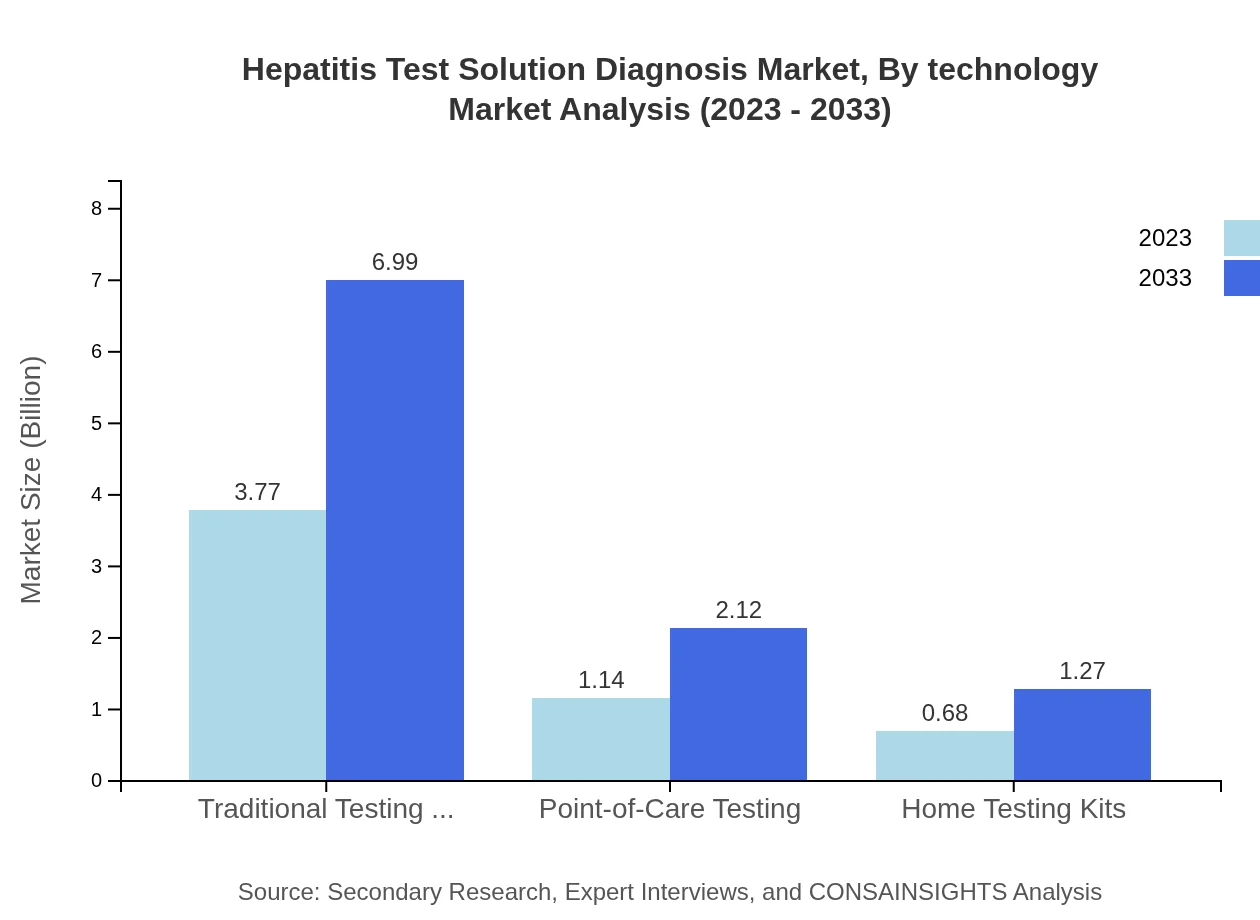
Hepatitis Test Solution Diagnosis Market Analysis By Technology
Technologically, traditional testing methods dominate with a share of 67.38%, set to grow from $3.77 billion in 2023 to $6.99 billion by 2033. Point-of-care testing, though currently at $1.14 billion, shows potential with expected expansion to $2.12 billion.
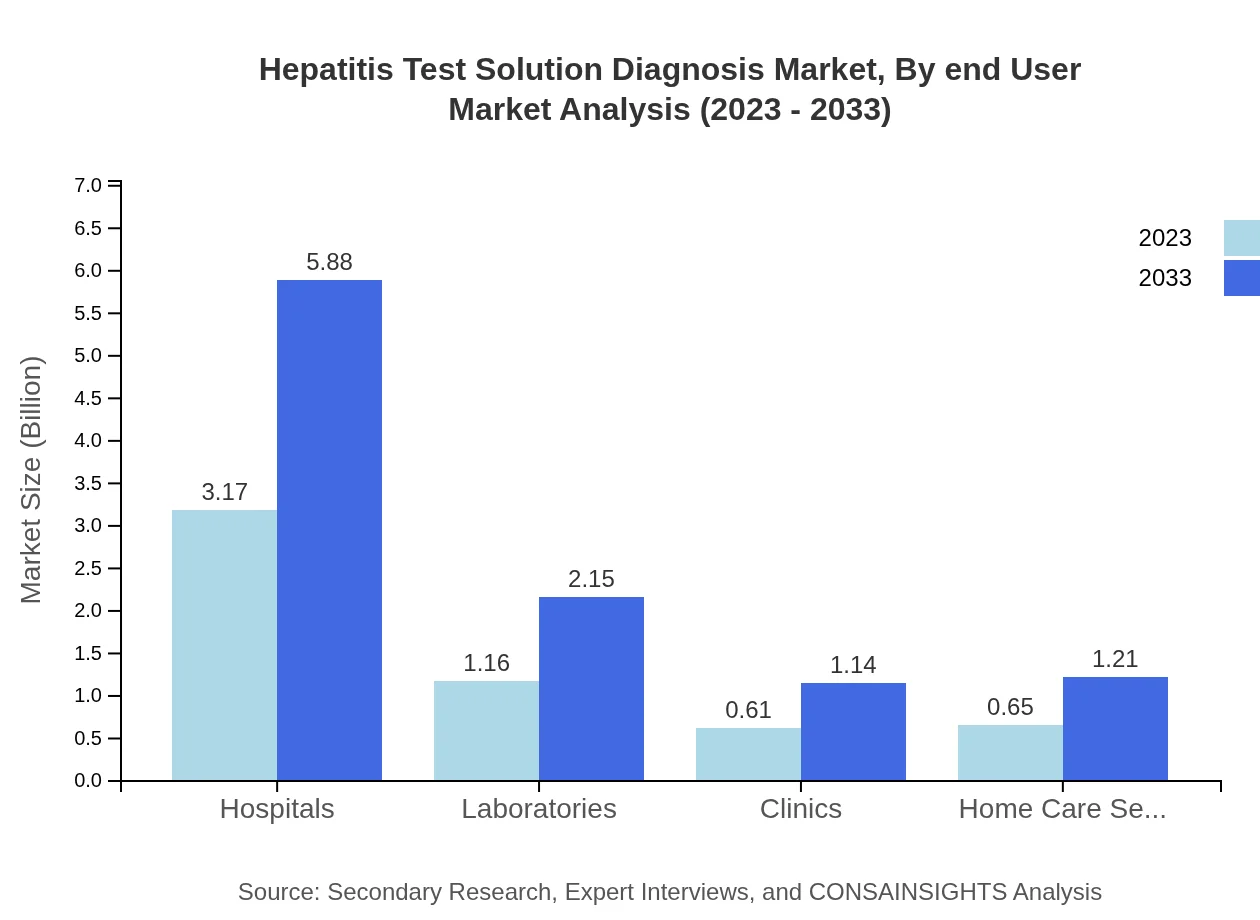
Hepatitis Test Solution Diagnosis Market Analysis By End User
Hospitals are leading end-users with a market share of 56.64%, increasing from $3.17 billion in 2023 to $5.88 billion by 2033. Laboratories follow, with a share of 20.76%, projected to rise from $1.16 billion to $2.15 billion. Clinical settings are anticipated to grow from $0.61 billion to $1.14 billion.
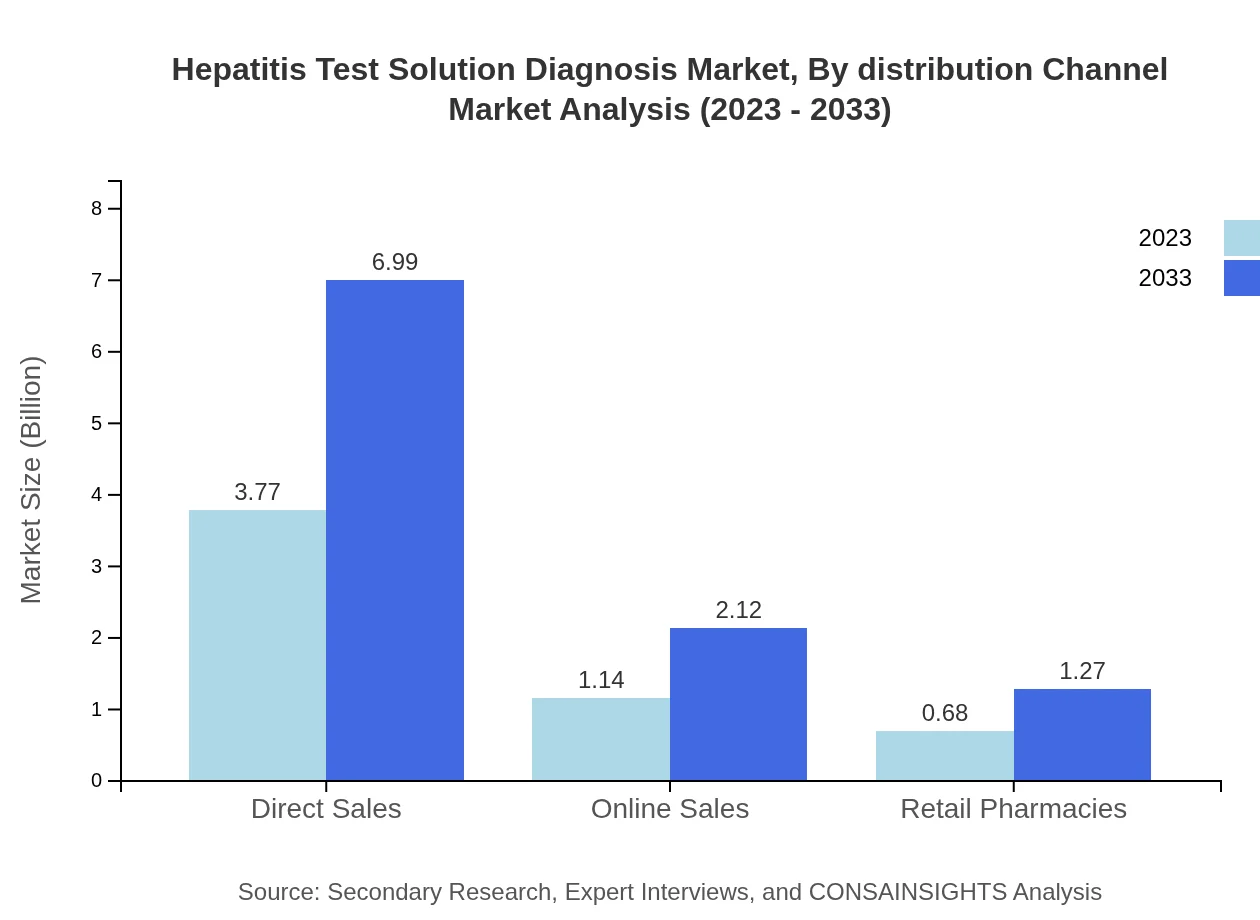
Hepatitis Test Solution Diagnosis Market Analysis By Distribution Channel
The distribution channels reveal that direct sales hold a significant market share of 67.38%, with values growing from $3.77 billion in 2023 to $6.99 billion by 2033. Online sales represent a growing channel, expected to rise from $1.14 billion to $2.12 billion.
Hepatitis Test Solution Diagnosis Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hepatitis Test Solution Diagnosis Industry
Roche Diagnostics:
Roche Diagnostics is a global leader in the healthcare sector, known for its advanced diagnostic solutions and innovative products that address various diseases, including Hepatitis.Abbott Laboratories:
Abbott Laboratories excels in providing comprehensive diagnostic products and services, contributing significantly to the Hepatitis testing sector through innovative technologies.Siemens Healthineers:
Siemens Healthineers offers a wide range of diagnostic solutions, and their commitment to advancing Hepatitis diagnostics ensures they remain a key player in the industry.bioMérieux:
bioMérieux specializes in microbiological testing and is dedicated to developing precise testing solutions for Hepatitis, enhancing patient care nationwide.We're grateful to work with incredible clients.









FAQs
What is the market size of hepatitis Test Solution Diagnosis?
The global Hepatitis Test Solution Diagnosis market was valued at approximately $5.6 billion in 2023, with a projected CAGR of 6.2% from 2023 to 2033. This growth reflects an increasing focus on hepatitis screening and diagnosis.
What are the key market players or companies in the hepatitis Test Solution Diagnosis industry?
Key players in the Hepatitis Test Solution Diagnosis industry include leading biotech firms and diagnostic companies specializing in infectious diseases. Notable companies invest heavily in research and development to offer advanced diagnostic tests tailored to hepatitis detection.
What are the primary factors driving the growth in the hepatitis test solution diagnosis industry?
The growth of the hepatitis test solution diagnosis industry is driven by factors such as rising hepatitis prevalence, increased awareness programs, technological advancements in diagnostics, and supportive government initiatives promoting routine hepatitis screening.
Which region is the fastest Growing in the hepatitis test solution diagnosis?
North America is currently the fastest-growing region in the hepatitis test solution diagnosis market, with anticipated growth from $1.82 billion in 2023 to $3.37 billion by 2033. High healthcare expenditure and advanced healthcare infrastructure contribute to this growth.
Does Consainsights provide customized market report data for the hepatitis test solution diagnosis industry?
Yes, Consainsights offers customized market reports tailored to specific client needs in the hepatitis test solution diagnosis sector. This includes tailored insights on market trends, regional analytics, and competitive landscapes based on customer requirements.
What deliverables can I expect from this hepatitis test solution diagnosis market research project?
Clients can expect comprehensive deliverables including detailed reports on market size, growth forecasts, regional analysis, insights on key players, and segment performance. Data visualization tools will also be provided for easier interpretation.
What are the market trends of hepatitis test solution diagnosis?
Current trends in the hepatitis test solution diagnosis market include the shift towards home testing kits, the use of point-of-care testing, and the integration of digital health solutions, which are revolutionizing traditional diagnostic methods for improved patient access.