Hepatitis Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: hepatitis-therapeutics
Hepatitis Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report on the Hepatitis Therapeutics market provides a comprehensive analysis of current trends, market size, segmentation, and regional insights from 2023 to 2033, offering valuable data for stakeholders in the healthcare industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
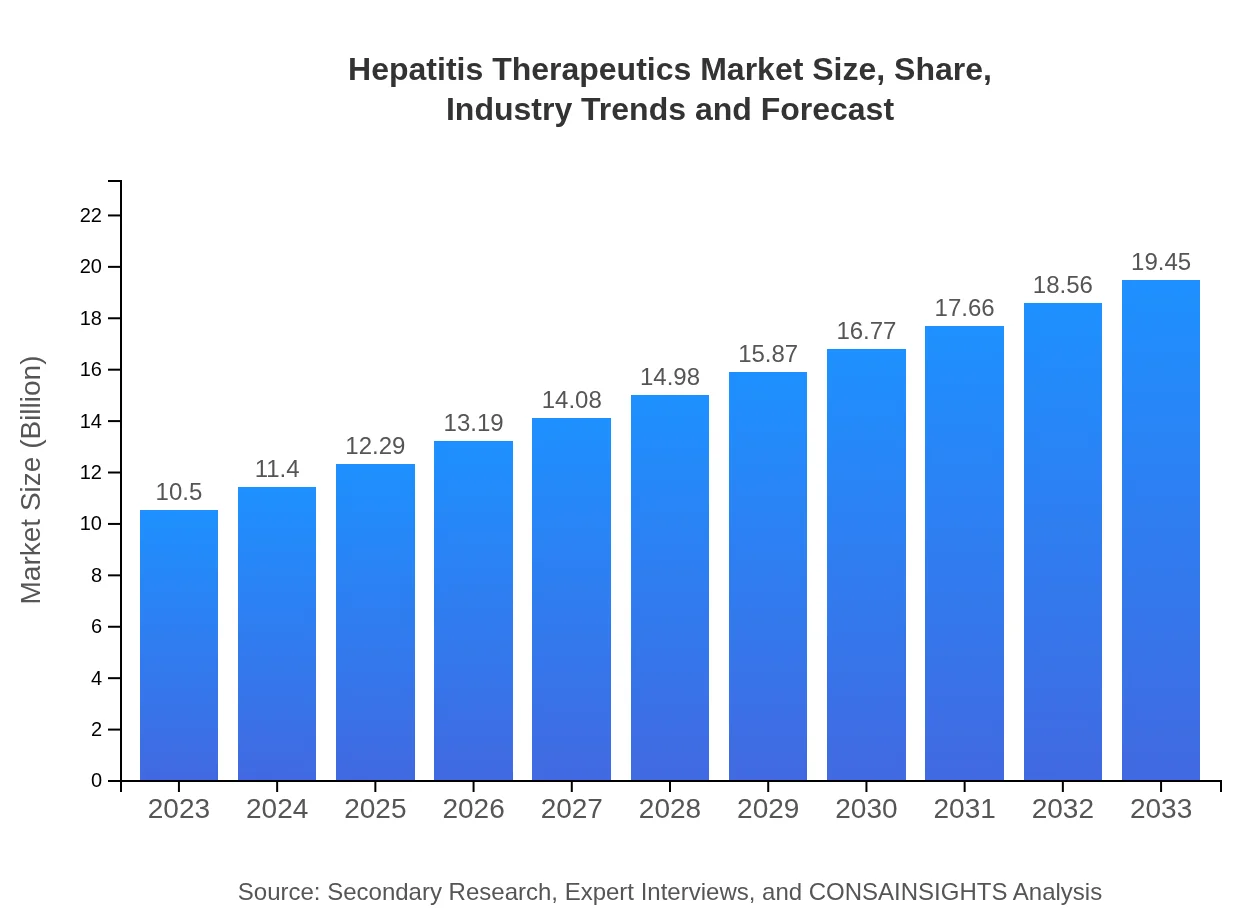
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $19.45 Billion |
| Top Companies | Gilead Sciences, Inc., AbbVie Inc., Merck & Co., Inc., GSK, Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Hepatitis Therapeutics Market Overview
Customize Hepatitis Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Hepatitis Therapeutics market size, growth, and forecasts.
- ✔ Understand Hepatitis Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hepatitis Therapeutics
What is the Market Size & CAGR of Hepatitis Therapeutics market in 2023?
Hepatitis Therapeutics Industry Analysis
Hepatitis Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hepatitis Therapeutics Market Analysis Report by Region
Europe Hepatitis Therapeutics Market Report:
The European Hepatitis Therapeutics market is projected to grow from $2.97 billion in 2023 to $5.50 billion by 2033. This growth is bolstered by robust healthcare policies, active research initiatives, and a well-established pharmaceutical sector focused on hepatitis treatment.Asia Pacific Hepatitis Therapeutics Market Report:
In the Asia Pacific region, the Hepatitis Therapeutics market is anticipated to grow from $2.14 billion in 2023 to $3.97 billion by 2033. This growth is driven by increasing prevalence rates of hepatitis, expanding healthcare access, and significant government initiatives aimed at vaccination and treatment.North America Hepatitis Therapeutics Market Report:
In North America, the market is expected to expand from $3.40 billion in 2023 to $6.30 billion by 2033, driven primarily by advanced healthcare systems, predominant pharmaceutical companies, and sustained research funding focused on hepatitis treatments.South America Hepatitis Therapeutics Market Report:
South America is projected to see growth in the Hepatitis Therapeutics market from $0.73 billion in 2023 to $1.36 billion in 2033. Factors such as rising awareness of viral hepatitis and increased healthcare funding contribute to this market expansion.Middle East & Africa Hepatitis Therapeutics Market Report:
The Middle East and Africa region are expected to witness growth from $1.26 billion in 2023 to $2.33 billion by 2033, largely due to increasing awareness campaigns about hepatitis prevention, improving healthcare infrastructure, and international partnerships focusing on viral hepatitis responses.Tell us your focus area and get a customized research report.
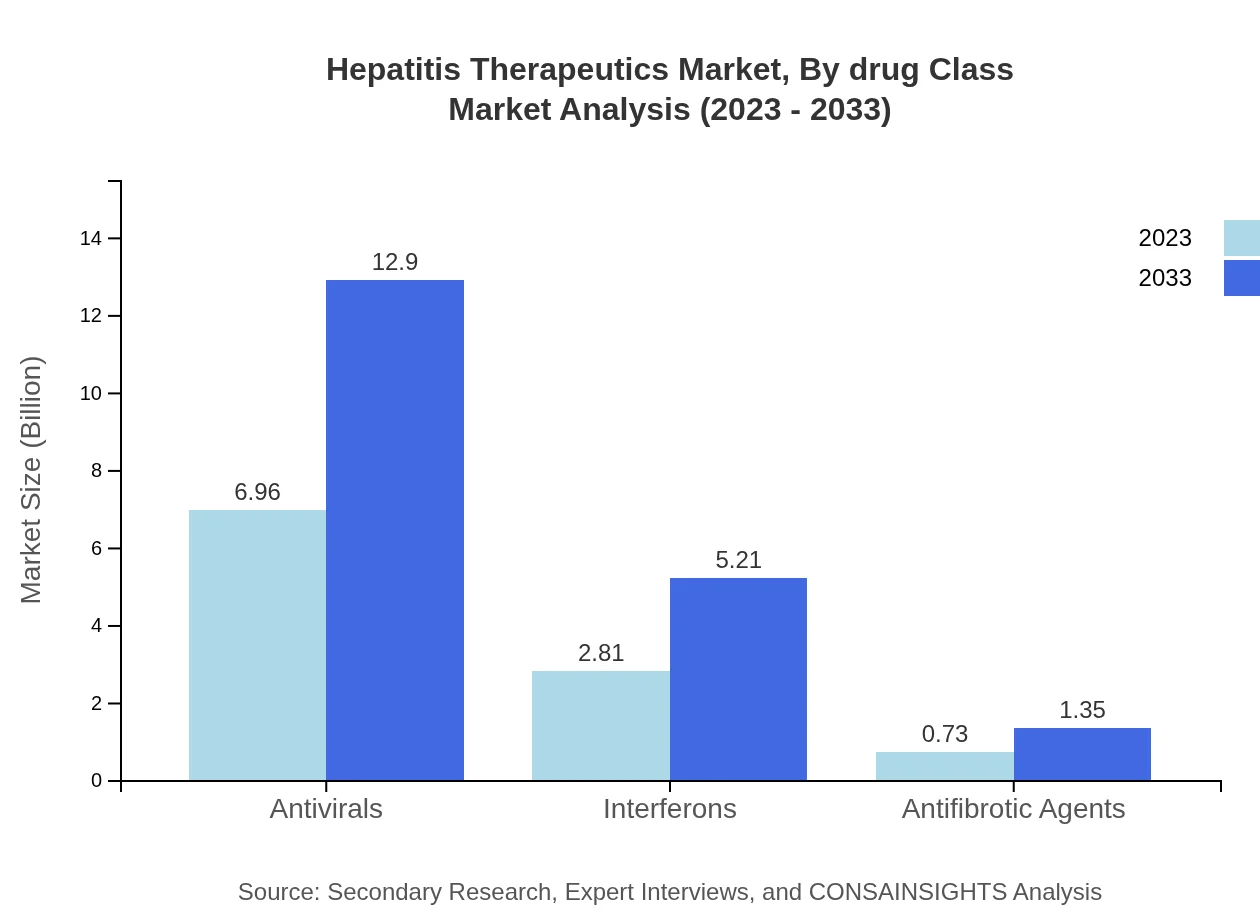
Hepatitis Therapeutics Market Analysis By Drug Class
The Hepatitis Therapeutics market showcases significant size variation across drug classes. Antivirals dominate the segment with a market size of $6.96 billion in 2023, expected to rise to $12.90 billion by 2033. This class is crucial for managing Hepatitis B and C. Interferons follow with a size of $2.81 billion in 2023, increasing to $5.21 billion by 2033. Antifibrotic agents currently contribute $0.73 billion, projected to reach $1.35 billion by 2033.
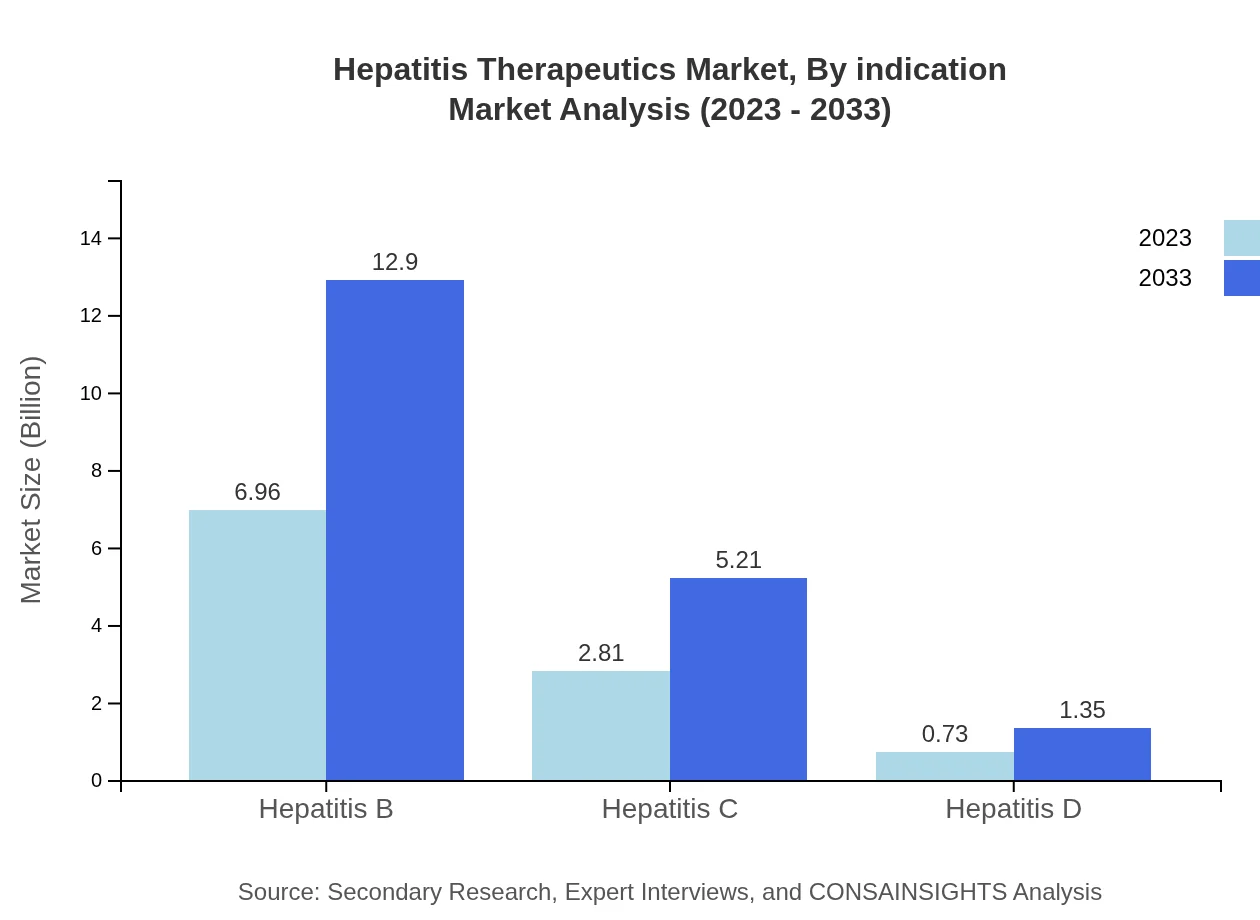
Hepatitis Therapeutics Market Analysis By Indication
Market segmentation by indication reflects the significant demand for effective treatments targeting various hepatitis types. In 2023, Hepatitis B therapeutics lead the market with $6.96 billion, expected to reach $12.90 billion in 2033. Hepatitis C follows at $2.81 billion, growing to $5.21 billion by 2033, while Hepatitis A and D account for smaller market shares but are critically important for specific patient demographics.
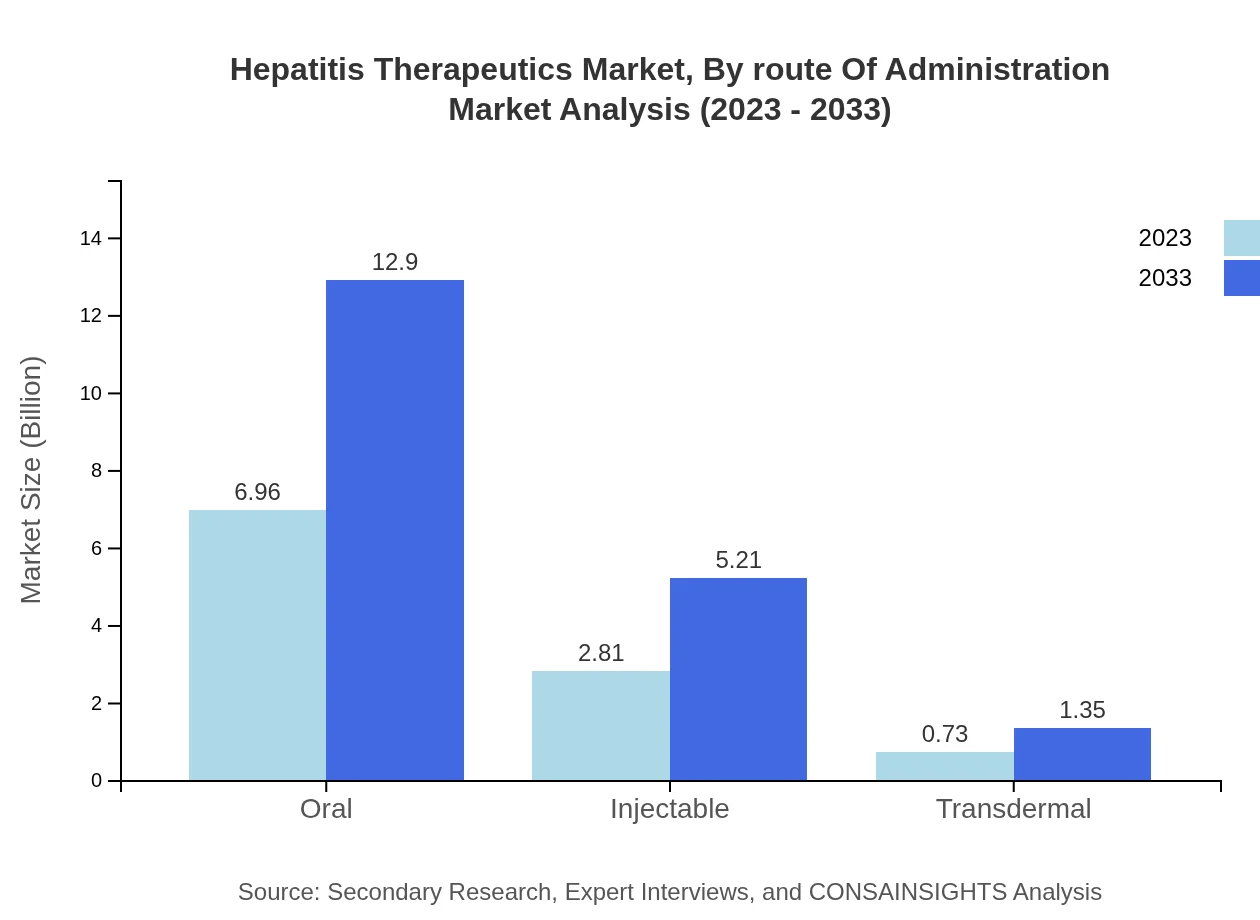
Hepatitis Therapeutics Market Analysis By Route Of Administration
Oral formulations dominate the Hepatitis Therapeutics market, valued at $6.96 billion in 2023 and projected to reach $12.90 billion by 2033. Injectable solutions are also significant, with a market size of $2.81 billion in 2023, growing to $5.21 billion by 2033. Transdermal delivery systems, though smaller in scope, show promise with a growth trajectory from $0.73 billion to $1.35 billion.
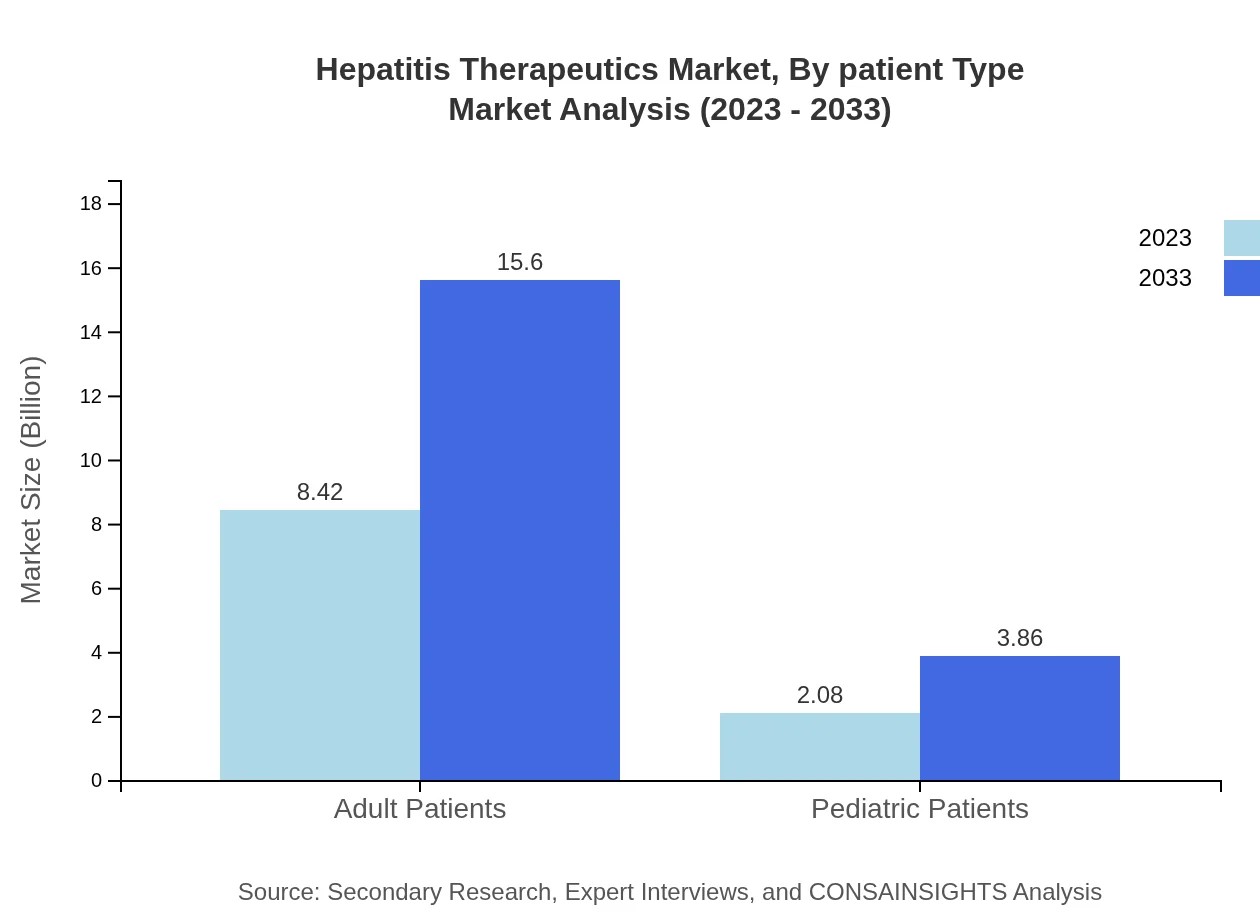
Hepatitis Therapeutics Market Analysis By Patient Type
The Hepatitis Therapeutics market categorized by patient type indicates a strong adult demographic, reflecting a size of $8.42 billion in 2023 and projected to reach $15.60 billion by 2033, capturing 80.18% of the market share. Pediatric patients account for a smaller segment, anticipated to grow from $2.08 billion to $3.86 billion by 2033.
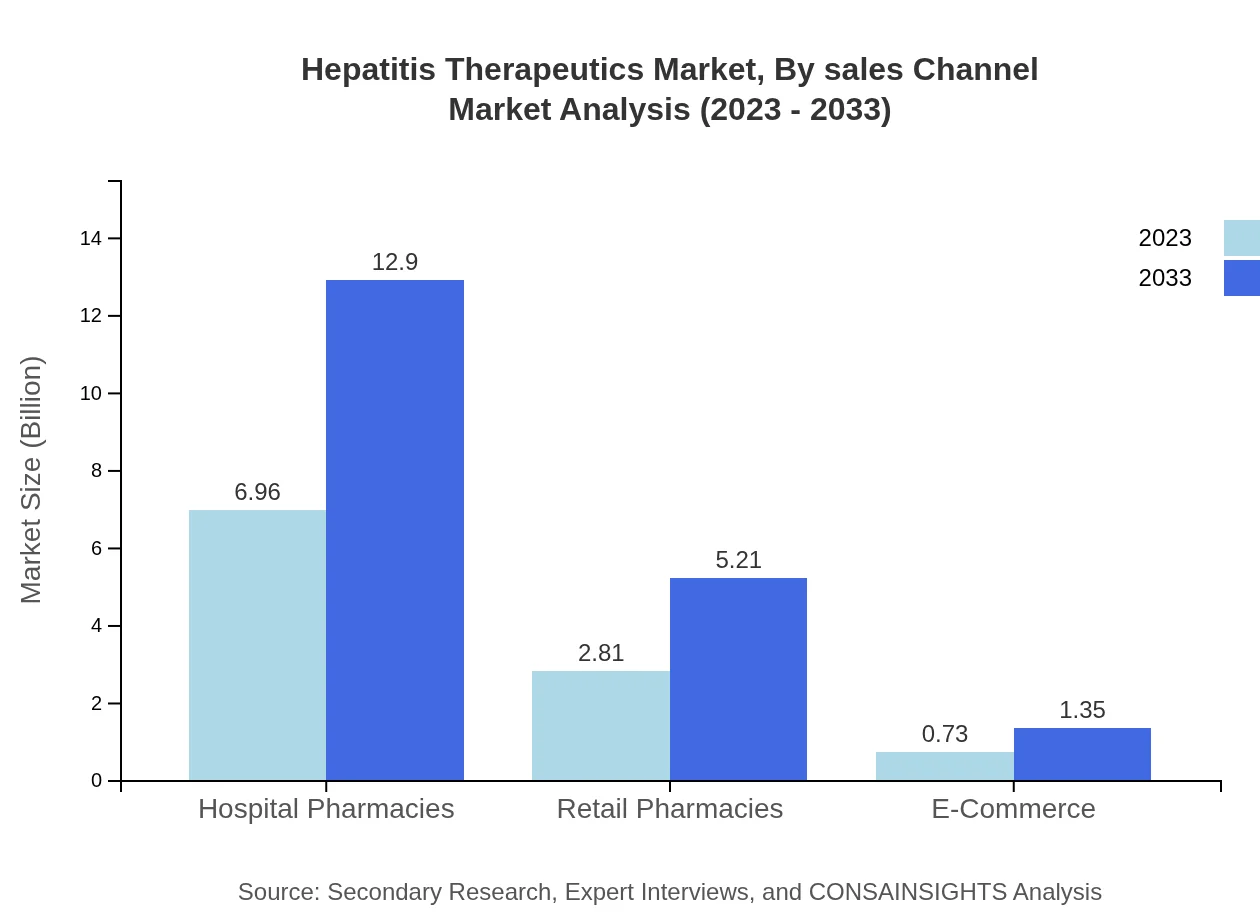
Hepatitis Therapeutics Market Analysis By Sales Channel
Hospital pharmacies lead the market representation with a size of $6.96 billion in 2023, expected to rise to $12.90 billion by 2033, capturing 66.31% of the market share. Retail pharmacies follow and are projected to grow from $2.81 billion to $5.21 billion, while e-commerce channels, although smaller at $0.73 billion, are anticipated to increase to $1.35 billion by 2033.
Hepatitis Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hepatitis Therapeutics Industry
Gilead Sciences, Inc.:
A leading biopharmaceutical company dedicated to the research and development of innovative medicines, particularly for viral infections including Hepatitis B and C.AbbVie Inc.:
AbbVie focuses on developing advanced therapies for chronic viral infections, striving to enhance treatment outcomes for Hepatitis patients globally.Merck & Co., Inc.:
Known for its robust portfolio in infectious diseases, Merck has pioneered treatments in Hepatitis B and C, showcasing significant contributions to therapeutic advancements.GSK:
GlaxoSmithKline is committed to addressing global health issues, including the development of effective vaccines for Hepatitis A and B.Bristol-Myers Squibb:
BMS is engaged in the development of innovative therapies targeting Hepatitis B, C, and associated complications, contributing to the market's evolution.We're grateful to work with incredible clients.









FAQs
What is the market size of hepatitis Therapeutics?
The hepatitis therapeutics market is valued at $10.5 billion in 2023, with a projected CAGR of 6.2% through 2033. This growth reflects advancements in treatment options and increasing prevalence of hepatitis infections globally.
What are the key market players or companies in this hepatitis Therapeutics industry?
Key players in the hepatitis therapeutics market include Gilead Sciences, AbbVie, and Merck & Co., among others. These companies lead in innovation, R&D efforts, and global market presence, contributing significantly to treatment advancements.
What are the primary factors driving the growth in the hepatitis therapeutics industry?
Growth in the hepatitis therapeutics industry is driven by increasing infection rates, rising healthcare expenditure, and extensive research in antiviral therapies. Additionally, awareness campaigns and government initiatives promote early screening and treatment access.
Which region is the fastest Growing in the hepatitis therapeutics market?
The Asia Pacific region is the fastest-growing in the hepatitis therapeutics market, with growth from $2.14 billion in 2023 to $3.97 billion by 2033. This expansion is fueled by increasing healthcare infrastructure and rising infection awareness.
Does ConsaInsights provide customized market report data for the hepatitis Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the hepatitis therapeutics industry. Clients can request data focusing on segments, geographical areas, and emerging trends for targeted insights.
What deliverables can I expect from this hepatitis Therapeutics market research project?
Deliverables from a hepatitis therapeutics market research project include comprehensive reports, segment analysis, geographical insights, trends, and data visualizations. These will provide in-depth understanding for strategic decision-making.
What are the market trends of hepatitis Therapeutics?
Market trends in hepatitis therapeutics include a shift towards oral antivirals, increasing adoption of combination therapies, and advancements in minimally invasive treatment options. A focus on patient-centric care is also emerging, tailoring therapies to individual needs.