Hereditary Angioedema Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: hereditary-angioedema-therapeutics
Hereditary Angioedema Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Hereditary Angioedema Therapeutics market, focusing on market size, trends, and forecasts from 2023 to 2033. Insights into regional dynamics, competitive landscape, and key industry players are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
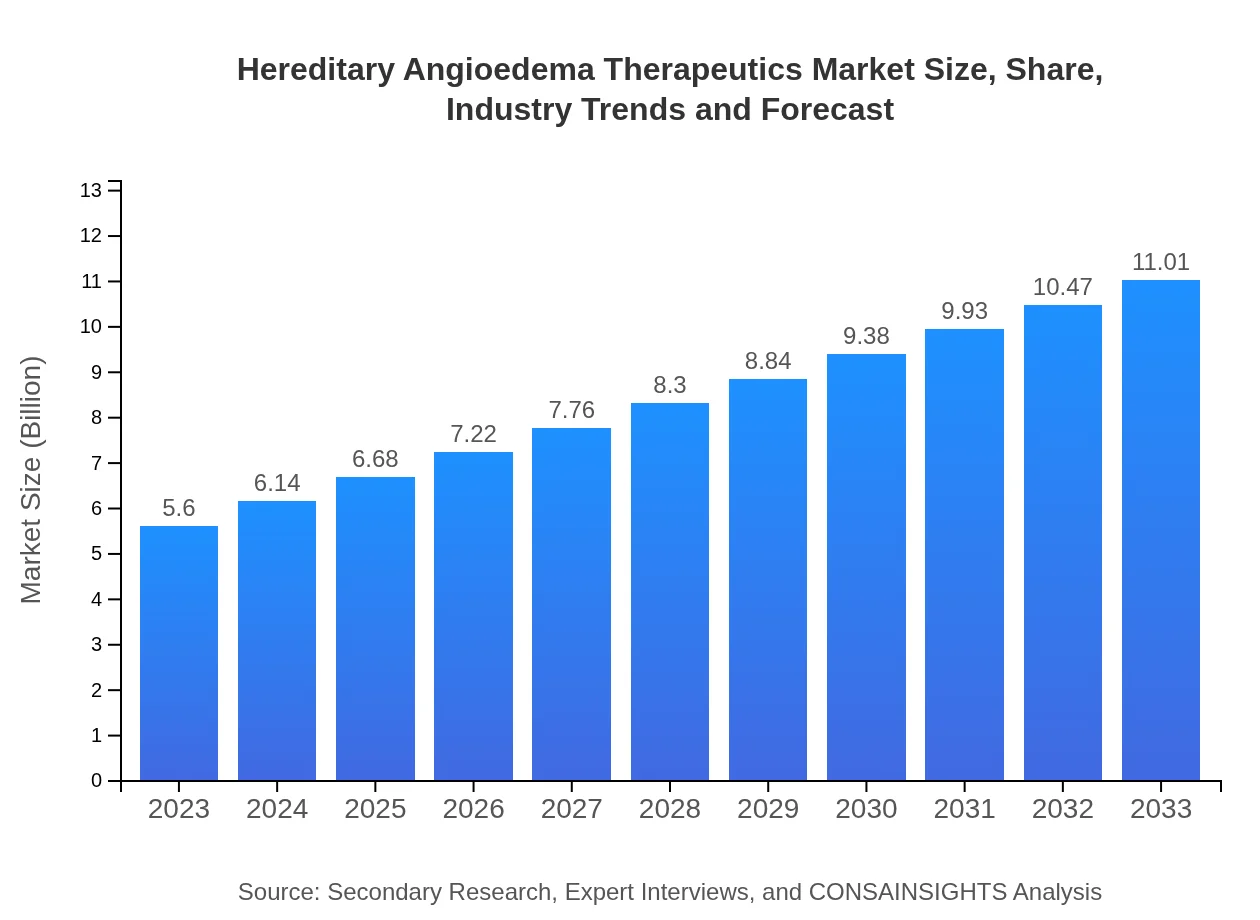
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $11.01 Billion |
| Top Companies | Takeda Pharmaceuticals, CSL Behring, Shire (part of Takeda) |
| Last Modified Date | 31 January 2026 |
Hereditary Angioedema Therapeutics Market Overview
Customize Hereditary Angioedema Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Hereditary Angioedema Therapeutics market size, growth, and forecasts.
- ✔ Understand Hereditary Angioedema Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hereditary Angioedema Therapeutics
What is the Market Size & CAGR of Hereditary Angioedema Therapeutics market in 2023?
Hereditary Angioedema Therapeutics Industry Analysis
Hereditary Angioedema Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hereditary Angioedema Therapeutics Market Analysis Report by Region
Europe Hereditary Angioedema Therapeutics Market Report:
In Europe, the HAE market is valued at USD 1.49 billion in 2023, projected to grow to USD 2.93 billion by 2033. The region benefits from high healthcare expenditure and a well-established regulatory framework for new therapies.Asia Pacific Hereditary Angioedema Therapeutics Market Report:
In the Asia Pacific region, the market is valued at USD 1.12 billion in 2023, expected to reach USD 2.21 billion by 2033. Rapid urbanization and improved healthcare infrastructure contribute to market growth in developing countries.North America Hereditary Angioedema Therapeutics Market Report:
North America leads the market, with a value of USD 2.13 billion in 2023, expecting to grow to USD 4.18 billion by 2033. The presence of numerous key players and advanced healthcare systems drive robust growth.South America Hereditary Angioedema Therapeutics Market Report:
The South American market for HAE therapeutics is valued at USD 0.28 billion in 2023 and projected to reach USD 0.55 billion by 2033. Increased awareness and healthcare access are pivotal to its expansion.Middle East & Africa Hereditary Angioedema Therapeutics Market Report:
The Middle East and Africa market stands at USD 0.58 billion in 2023, with expectations to reach USD 1.14 billion by 2033. Increased healthcare investments and awareness programs are significant contributors to market growth.Tell us your focus area and get a customized research report.
Hereditary Angioedema Therapeutics Market Analysis By Therapy Type
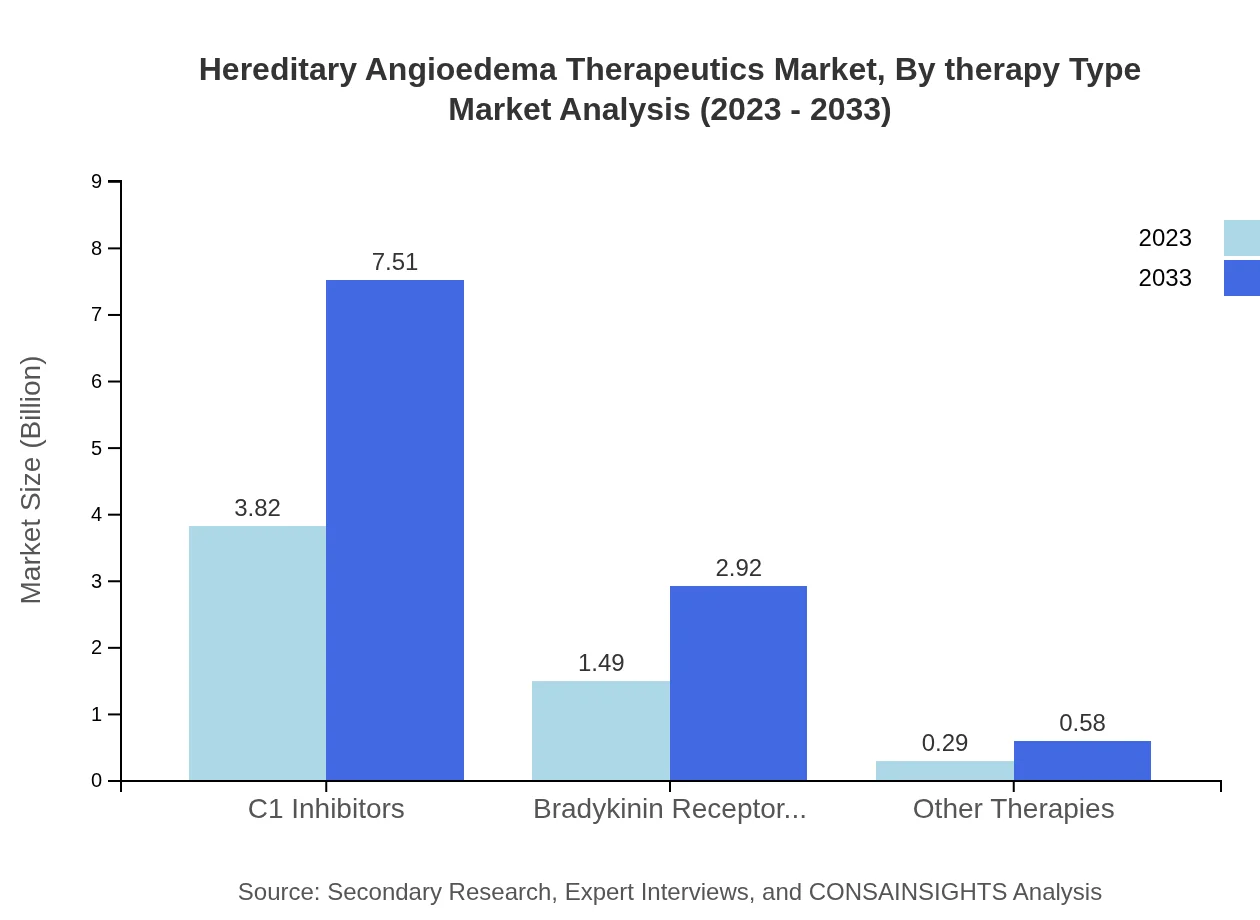
In the therapeutics market for hereditary angioedema, C1 inhibitors dominate with a market size of USD 3.82 billion in 2023, projected to increase to USD 7.51 billion by 2033, holding a 68.19% market share. Bradykinin receptor antagonists account for USD 1.49 billion in 2023, expected to grow to USD 2.92 billion by 2033, capturing 26.57% of the market. Other therapies, including Acquired Angioedema treatments, show minor but growing contributions.
Hereditary Angioedema Therapeutics Market Analysis By Route Of Administration
Subcutaneous injections currently dominate the market with a size of USD 3.82 billion in 2023, predicted to rise to USD 7.51 billion by 2033, constituting 68.19% of the administration methods. Intravenous injections follow, valued at USD 1.49 billion in 2023 and estimated to reach USD 2.92 billion by 2033, holding a 26.57% share. Oral administration remains a smaller segment, with a size of USD 0.29 billion in 2023, likely to increase to USD 0.58 billion by 2033.
Hereditary Angioedema Therapeutics Market Analysis By Indication
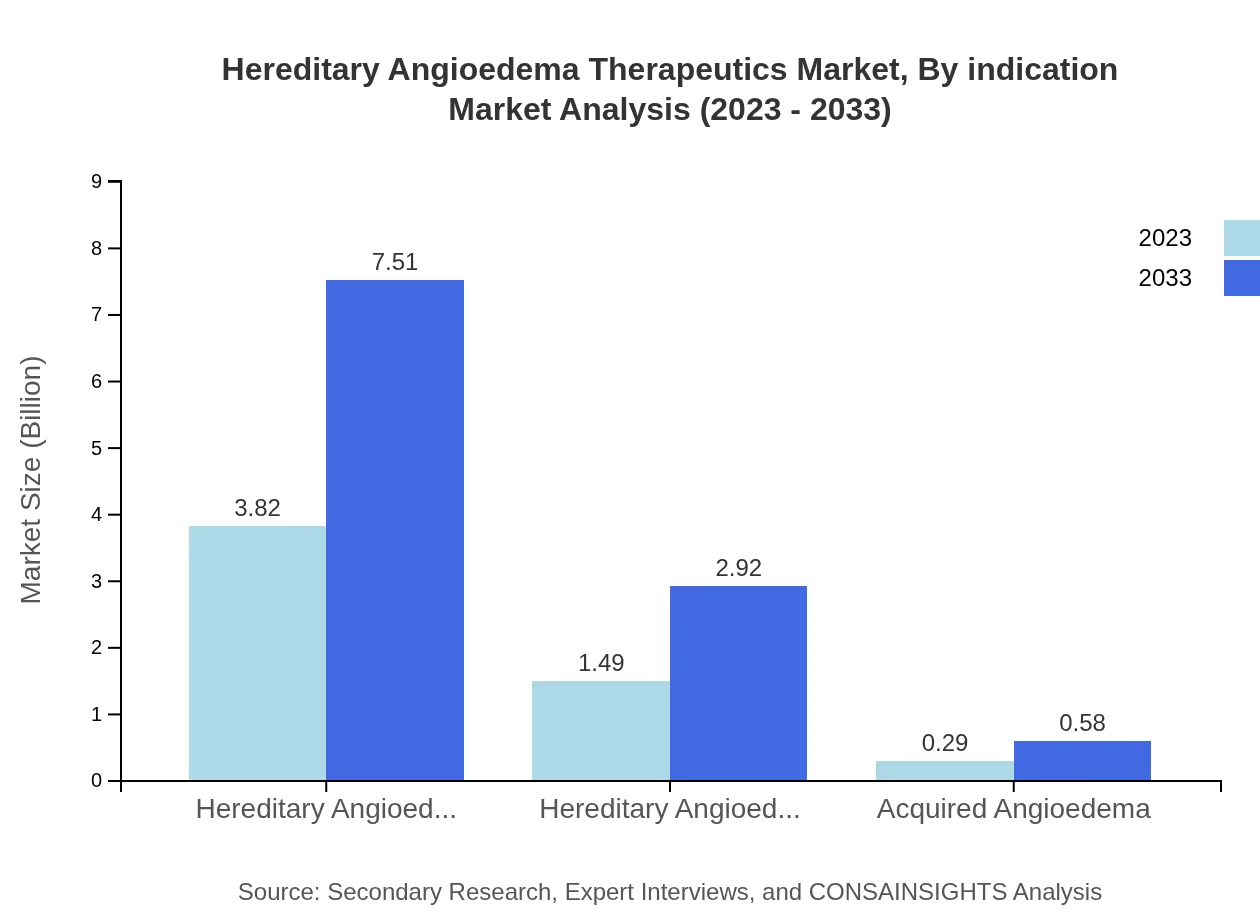
The market for Hereditary Angioedema is distinguished by type, with Hereditary Angioedema Type I at USD 3.82 billion in 2023 and expected to grow to USD 7.51 billion by 2033. Type II also shows potential, expected to grow from USD 1.49 billion to USD 2.92 billion over the same period. Acquired Angioedema, while smaller, is projected to rise from USD 0.29 billion in 2023 to USD 0.58 billion by 2033.
Hereditary Angioedema Therapeutics Market Analysis By End User
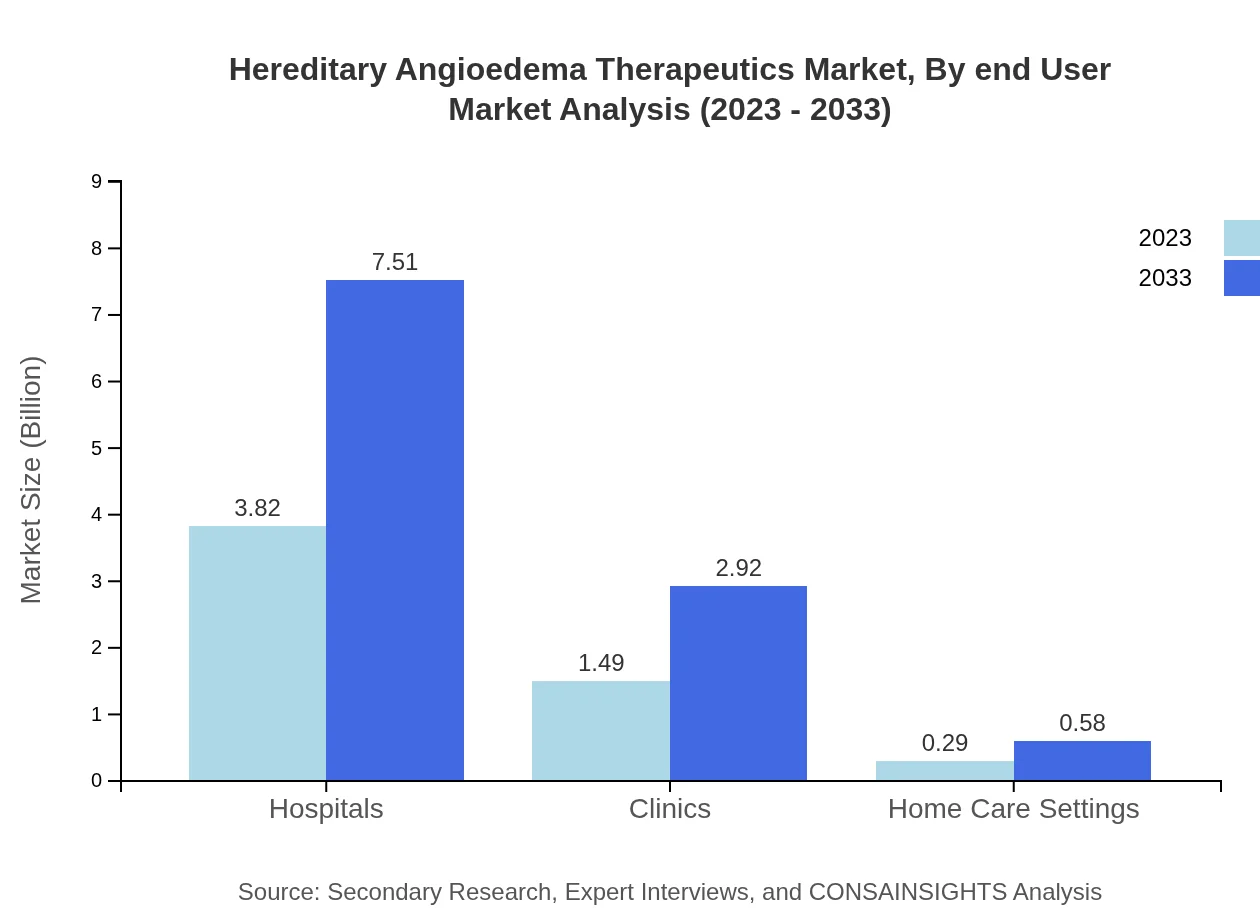
Hospitals are the primary end-users in this market, accounting for USD 3.82 billion in 2023 and likely to reach USD 7.51 billion by 2033, maintaining a 68.19% market share. Clinics represent another significant segment, with a size of USD 1.49 billion in 2023, projected to reach USD 2.92 billion by 2033, which corresponds to a 26.57% share. Home care settings also contribute, with values incrementing from USD 0.29 billion to USD 0.58 billion by 2033.
Hereditary Angioedema Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hereditary Angioedema Therapeutics Industry
Takeda Pharmaceuticals:
A leader in WFH (Hereditary Angioedema) therapies, Takeda provides advanced C1 esterase inhibitors and is at the forefront of HAE treatment innovation.CSL Behring:
Known for its extensive product line for plasma-derived therapies and innovative treatments for rare genetic disorders, including various HAE therapeutic options.Shire (part of Takeda):
Shire was pivotal in developing therapies that focus on the unique needs of HAE patients and was acquired by Takeda to enhance its portfolio.We're grateful to work with incredible clients.









FAQs
What is the market size of hereditary angioedema therapeutics?
The hereditary angioedema therapeutics market is valued at approximately $5.6 billion in 2023, with an anticipated CAGR of 6.8% from 2023 to 2033, indicating robust growth and a significant future market potential.
What are the key market players or companies in this hereditary angioedema therapeutics industry?
Key players in the hereditary angioedema therapeutics market include Shire plc (now part of Takeda), CSL Behring, and BioCryst Pharmaceuticals, which have developed innovative treatment modalities and therapies for managing HAE symptoms effectively.
What are the primary factors driving the growth in the hereditary angioedema therapeutics industry?
Key drivers for market growth include rising awareness of hereditary angioedema, an increase in the prevalence of the disorder, advancements in treatment options, and a growing focus on research to improve patient care and therapeutic outcomes.
Which region is the fastest Growing in the hereditary angioedema therapeutics market?
The Asia-Pacific region is identified as the fastest-growing market for hereditary angioedema therapeutics, with the market projected to increase from $1.12 billion in 2023 to $2.21 billion by 2033.
Does ConsaInsights provide customized market report data for the hereditary angioedema therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the hereditary angioedema therapeutics industry, ensuring clients receive insights that align with their unique research requirements.
What deliverables can I expect from this hereditary angioedema therapeutics market research project?
Deliverables include detailed market reports, trend analyses, competitive landscape assessments, regional market breakdowns, and data on market sizes across various segments, offering a comprehensive view of the industry.
What are the market trends of hereditary angioedema therapeutics?
Current trends include increasing adoption of innovative therapies, a shift towards personalized medicine, and an upsurge in clinical trials focused on gene therapy and new treatment modalities for hereditary angioedema management.