Hernia Mesh Devices Market Report
Published Date: 31 January 2026 | Report Code: hernia-mesh-devices
Hernia Mesh Devices Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the Hernia Mesh Devices market, detailing the current state and future prospects between 2023 to 2033. It includes insights on market size, segmentation, industry analysis, regional performance, and trends shaping the market landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
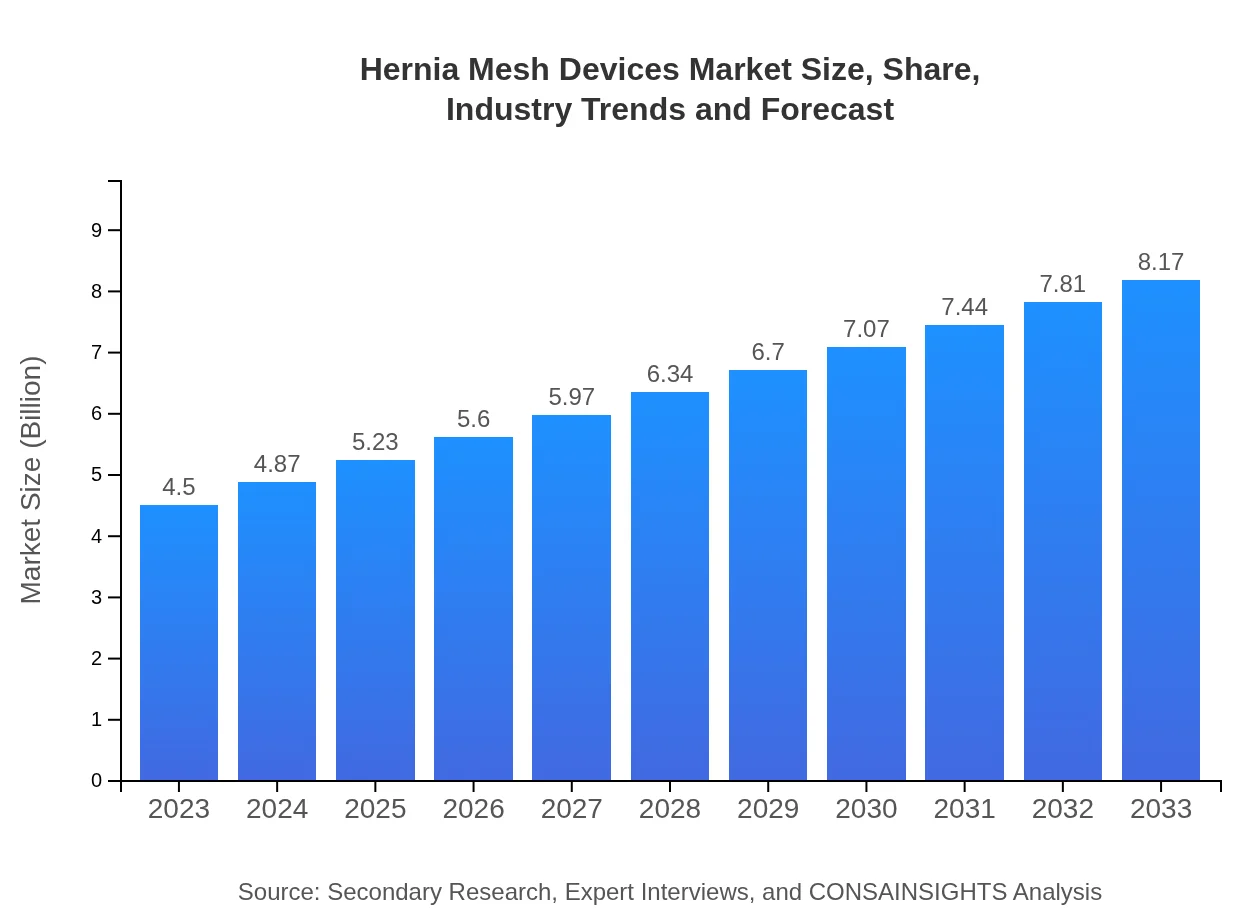
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6% |
| 2033 Market Size | $8.17 Billion |
| Top Companies | Medtronic , Ethicon (Johnson & Johnson), Atrium Medical Corporation, Bard (C.R. Bard) |
| Last Modified Date | 31 January 2026 |
Hernia Mesh Devices Market Overview
Customize Hernia Mesh Devices Market Report market research report
- ✔ Get in-depth analysis of Hernia Mesh Devices market size, growth, and forecasts.
- ✔ Understand Hernia Mesh Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hernia Mesh Devices
What is the Market Size & CAGR of Hernia Mesh Devices market?
Hernia Mesh Devices Industry Analysis
Hernia Mesh Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hernia Mesh Devices Market Analysis Report by Region
Europe Hernia Mesh Devices Market Report:
In Europe, the market size is forecasted to grow from $1.39 billion in 2023 to $2.53 billion by 2033, driven by chronic disease prevalence and a robust healthcare system promoting surgical interventions.Asia Pacific Hernia Mesh Devices Market Report:
In the Asia Pacific region, the Hernia Mesh Devices market was valued at $0.83 billion in 2023, projected to grow to $1.52 billion by 2033. Factors such as increasing surgical procedures and favorable healthcare initiatives are driving this growth.North America Hernia Mesh Devices Market Report:
The North American market, valued at $1.69 billion in 2023, is anticipated to reach $3.06 billion by 2033, fueled by the prevalence of hernia surgeries and technologically advanced products.South America Hernia Mesh Devices Market Report:
South America’s market for Hernia Mesh Devices stood at $0.39 billion in 2023, expected to rise to $0.71 billion by 2033. Increased health awareness and rising disposable incomes are likely contributing factors.Middle East & Africa Hernia Mesh Devices Market Report:
The market in the Middle East and Africa is smaller, starting at $0.20 billion in 2023 and expected to reach $0.37 billion by 2033. Poverty and healthcare access challenges are slowing growth, but initiatives to improve healthcare infrastructure show promise.Tell us your focus area and get a customized research report.
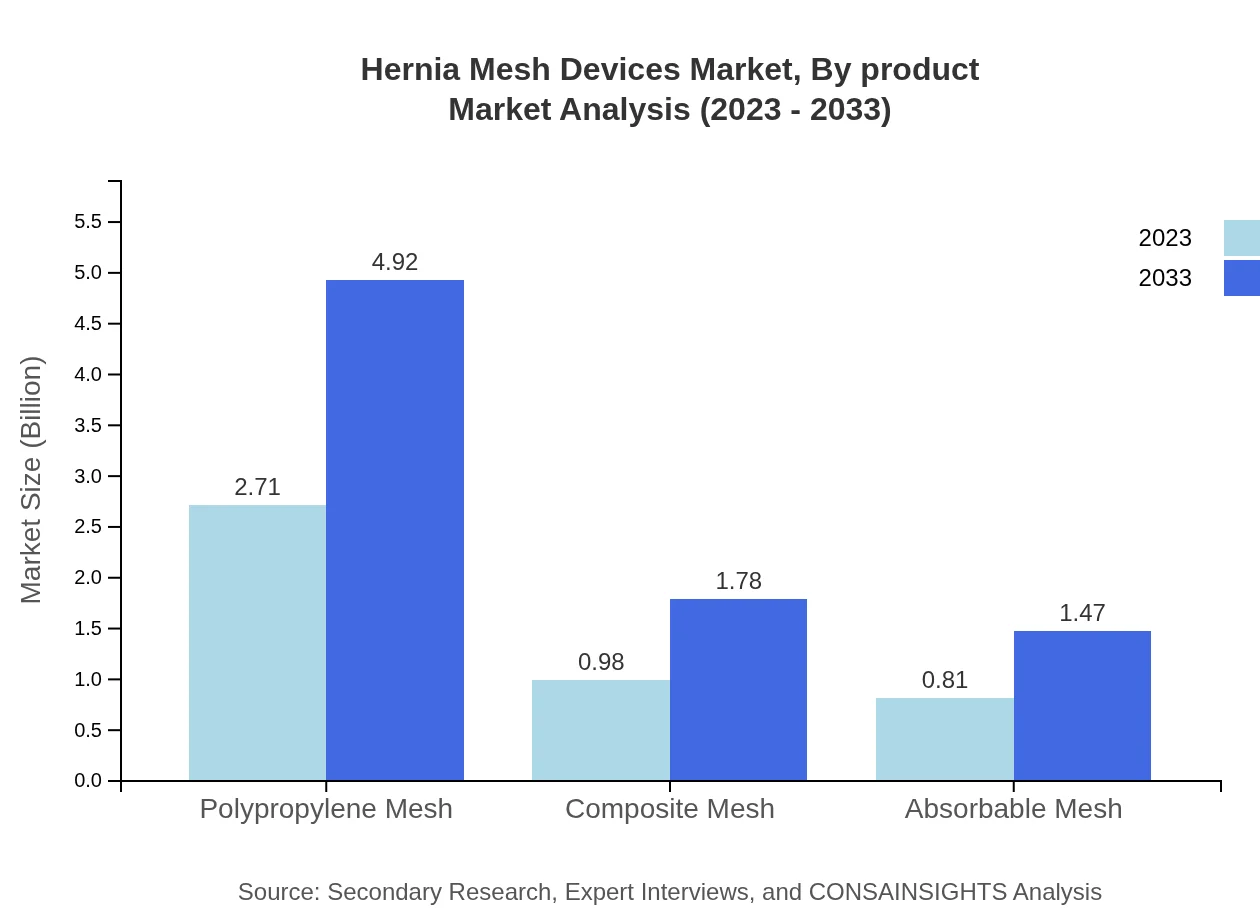
Hernia Mesh Devices Market Analysis By Product
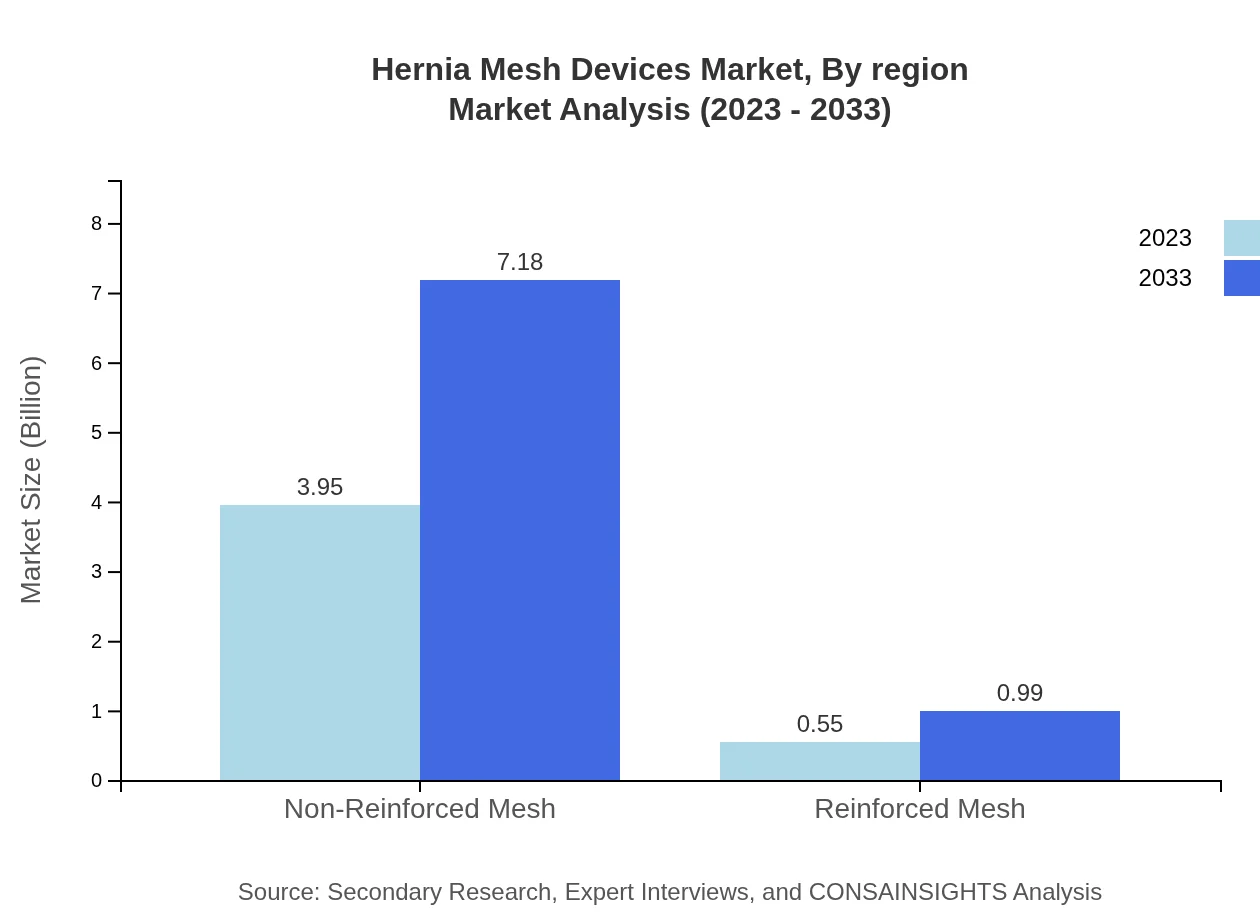
The Hernia Mesh Devices market by product includes non-reinforced mesh, reinforced mesh, polypropylene mesh, composite mesh, and absorbable mesh. Non-reinforced mesh dominates the market with a size of approximately $3.95 billion in 2023, expected to grow to $7.18 billion by 2033, capturing 87.84% market share. In contrast, reinforced mesh is valued at $0.55 billion in 2023, likely to reach $0.99 billion by 2033, holding 12.16% share. The polypropylene segment, integral to modern surgical techniques, is projected to grow significantly from $2.71 billion in 2023 to $4.92 billion in 2033.
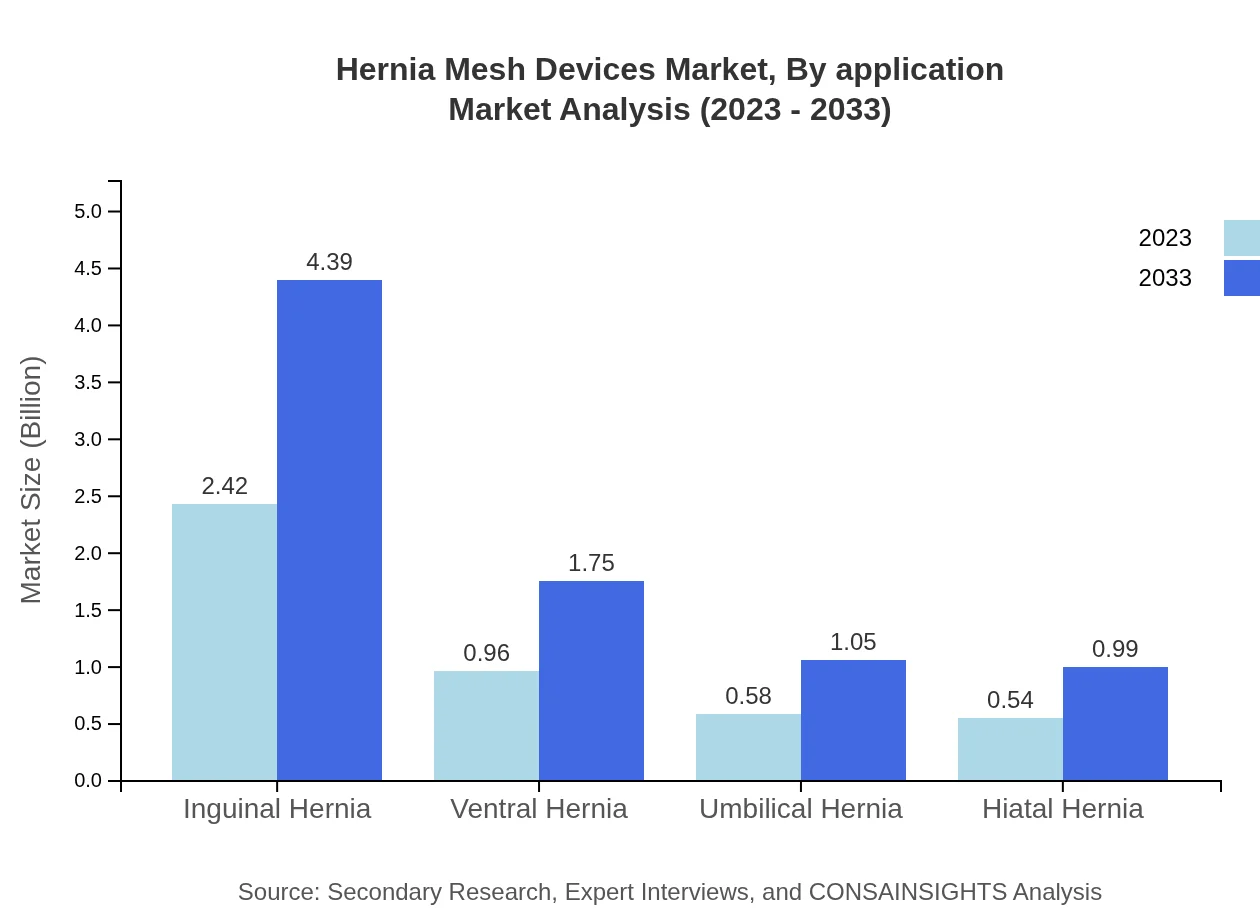
Hernia Mesh Devices Market Analysis By Application
The applications of Hernia Mesh Devices focus on different types of hernias, such as inguinal, ventral, umbilical, and hiatal. Inguinal hernia repair represents the largest market share, with a size of $2.42 billion in 2023 to expand to $4.39 billion in 2033, representing 53.74% of the market. Ventral hernia applications are valued at $0.96 billion in 2023 with an estimate of $1.75 billion by 2033, accounting for 21.35%. Each application reflects significant surgical procedures translating into a robust market for specialized products.
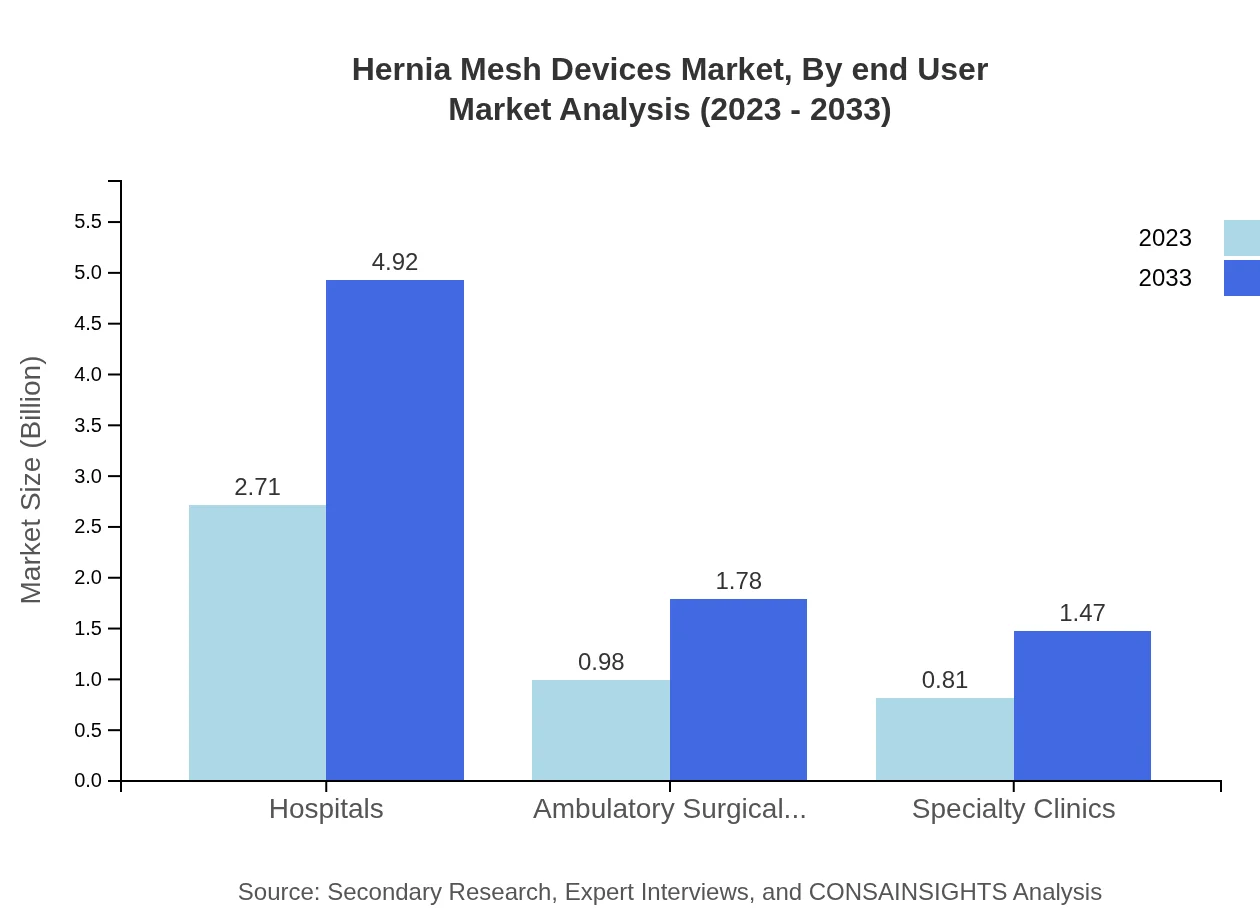
Hernia Mesh Devices Market Analysis By End User
The key end-users of Hernia Mesh Devices include hospitals, ambulatory surgical centers, and specialty clinics. The hospital segment dominates the market, growing from $2.71 billion in 2023 to $4.92 billion by 2033, comprising 60.22% of total market share. Ambulatory surgical centers are poised to increase from $0.98 billion to $1.78 billion during the same period, holding 21.75% of the market, while specialty clinics contribute $0.81 billion in 2023 projected to rise to $1.47 billion with an 18.03% share.
Hernia Mesh Devices Market Analysis By Region
The regional analysis of Hernia Mesh Devices encompasses diverse geographic markets with specific growth trajectories influenced by various factors. North America and Europe remain crucial due to advanced healthcare systems, surpassing other regions in market size. Conversely, Asia Pacific exhibits significant growth prospects due to increasing surgical innovations and healthcare improvement initiatives.
Hernia Mesh Devices Market Analysis By Material
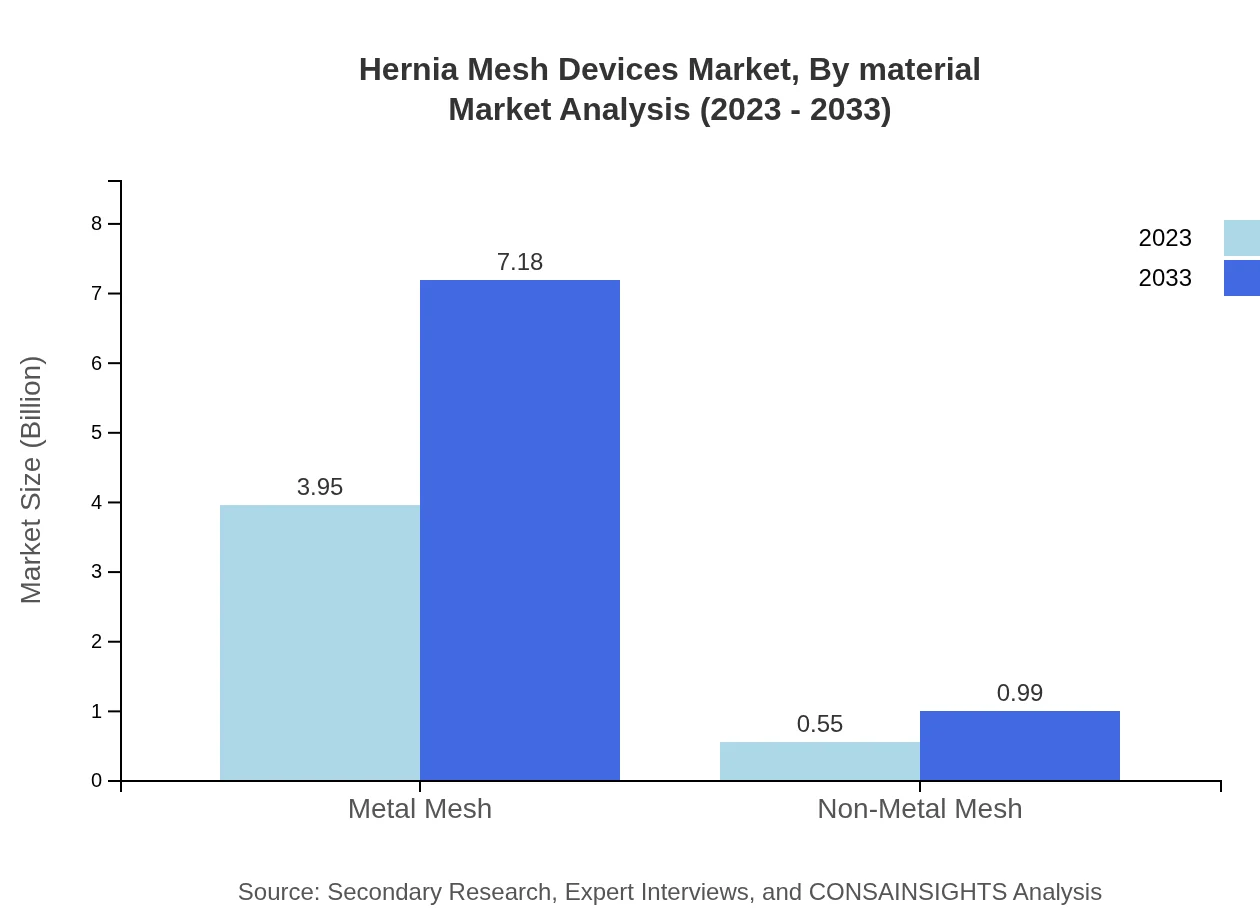
Hernia Mesh Devices are segmented based on material into metal and non-metal meshes. Metal mesh leads the segment with a size of $3.95 billion in 2023 projected to reach $7.18 billion by 2033, constituting 87.84% of the market. Non-metal mesh, while smaller at $0.55 billion in 2023, is expected to grow to $0.99 billion marked by 12.16% market share. This segmentation reflects preferences in surgical approaches and continued innovation in materials for enhanced patient outcomes.
Hernia Mesh Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hernia Mesh Devices Industry
Medtronic :
Medtronic is a leading global healthcare solutions company committed to innovation and patient care, providing a wide range of surgical products, including advanced hernia mesh devices.Ethicon (Johnson & Johnson):
Ethicon is known for its high-quality surgical products and innovative approaches in hernia repair, focusing on both mesh and suture technologies.Atrium Medical Corporation:
Atrium Medical specializes in developing innovative mesh products for surgical procedures, emphasizing ease of use and enhanced patient outcomes.Bard (C.R. Bard):
Bard has a robust portfolio of surgical products, including advanced hernia repair solutions, driven by extensive research and product evolution.We're grateful to work with incredible clients.









FAQs
What is the market size of hernia Mesh Devices?
The global hernia mesh devices market is estimated to be valued at approximately $4.5 billion in 2023, with a projected compound annual growth rate (CAGR) of 6% by 2033. This growth reflects increasing surgical interventions and advancements in mesh technology.
What are the key market players or companies in this hernia Mesh Devices industry?
Key players in the hernia mesh devices market include Medtronic, Boston Scientific, Ethicon (Johnson & Johnson), Atrium Medical, and Cook Medical. These companies lead in innovation and market share, offering a range of mesh products to meet diverse surgical needs.
What are the primary factors driving the growth in the hernia Mesh Devices industry?
Growth in the hernia mesh devices market is driven by rising surgical procedures, advancements in medical technology, increased awareness of hernia treatment options, and the growing incidence of hernias due to aging populations and obesity.
Which region is the fastest Growing in the hernia Mesh Devices?
The fastest-growing region in the hernia mesh devices market is North America, projected to reach $3.06 billion by 2033, up from $1.69 billion in 2023. This growth is fueled by high healthcare expenditure and rapid adoption of advanced medical devices.
Does ConsaInsights provide customized market report data for the hernia Mesh Devices industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the hernia mesh devices industry, enabling detailed insights into market trends, player strategies, and demographic specifics.
What deliverables can I expect from this hernia Mesh Devices market research project?
Expect deliverables such as detailed market analysis, segmentation insights, competitive landscape evaluation, regional performance data, and trends forecast. These insights can guide strategic decision-making and investment planning.
What are the market trends of hernia Mesh Devices?
Key market trends include the increasing adoption of minimally invasive surgical techniques, growth in outpatient procedures, advancements in bioengineered mesh materials, and a focus on patient safety and outcomes driving innovation in product design.