Hiv Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: hiv-therapeutics
Hiv Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the HIV therapeutics market, including trends, market size, and forecasts for the period 2023 to 2033, along with pivotal insights into the segmentation, regional analysis, and industry leaders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
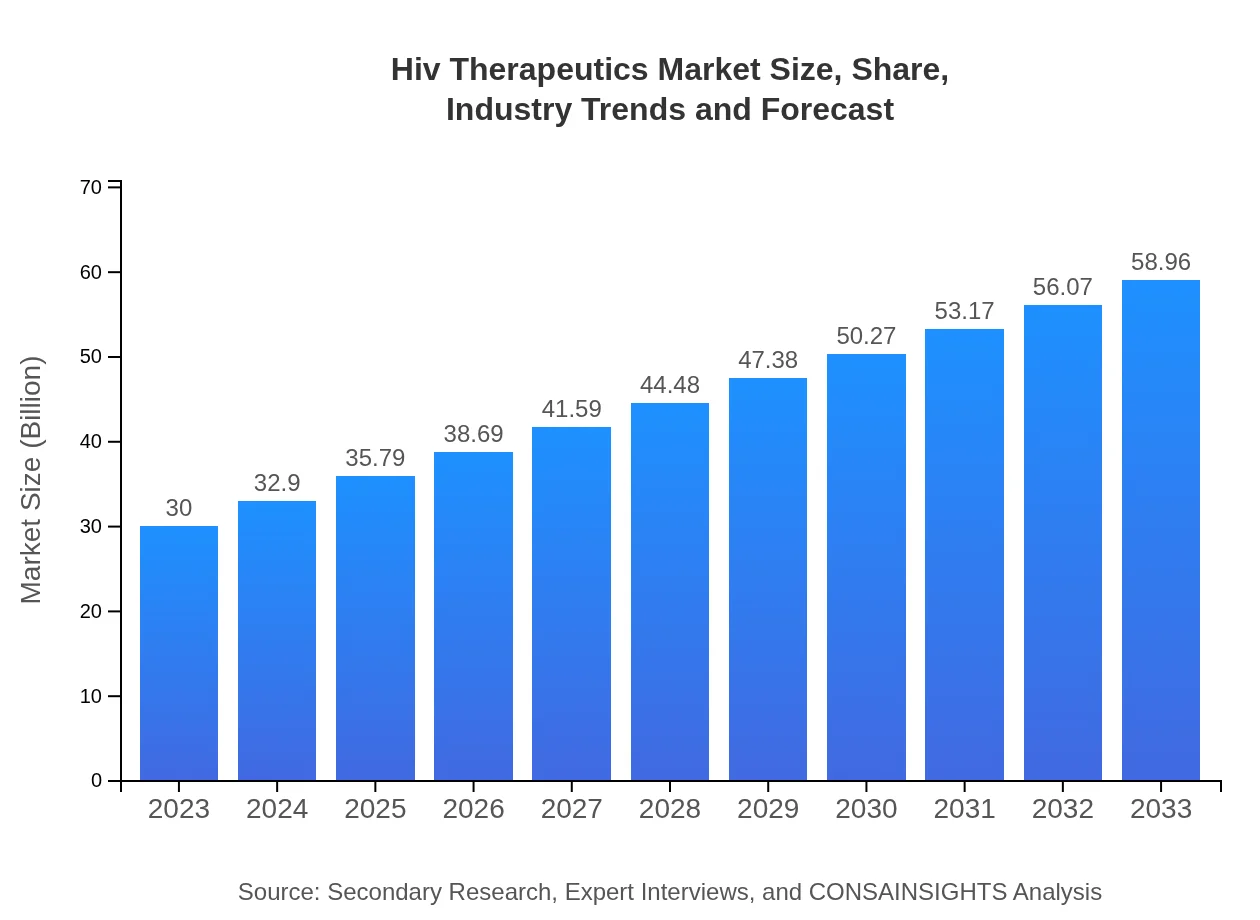
| 2023 Market Size | $30.00 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $58.96 Billion |
| Top Companies | Gilead Sciences, Viiv Healthcare, Johnson & Johnson, Merck & Co. |
| Last Modified Date | 31 January 2026 |
HIV Therapeutics Market Overview
Customize Hiv Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Hiv Therapeutics market size, growth, and forecasts.
- ✔ Understand Hiv Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hiv Therapeutics
What is the Market Size & CAGR of HIV Therapeutics market in 2023?
HIV Therapeutics Industry Analysis
HIV Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
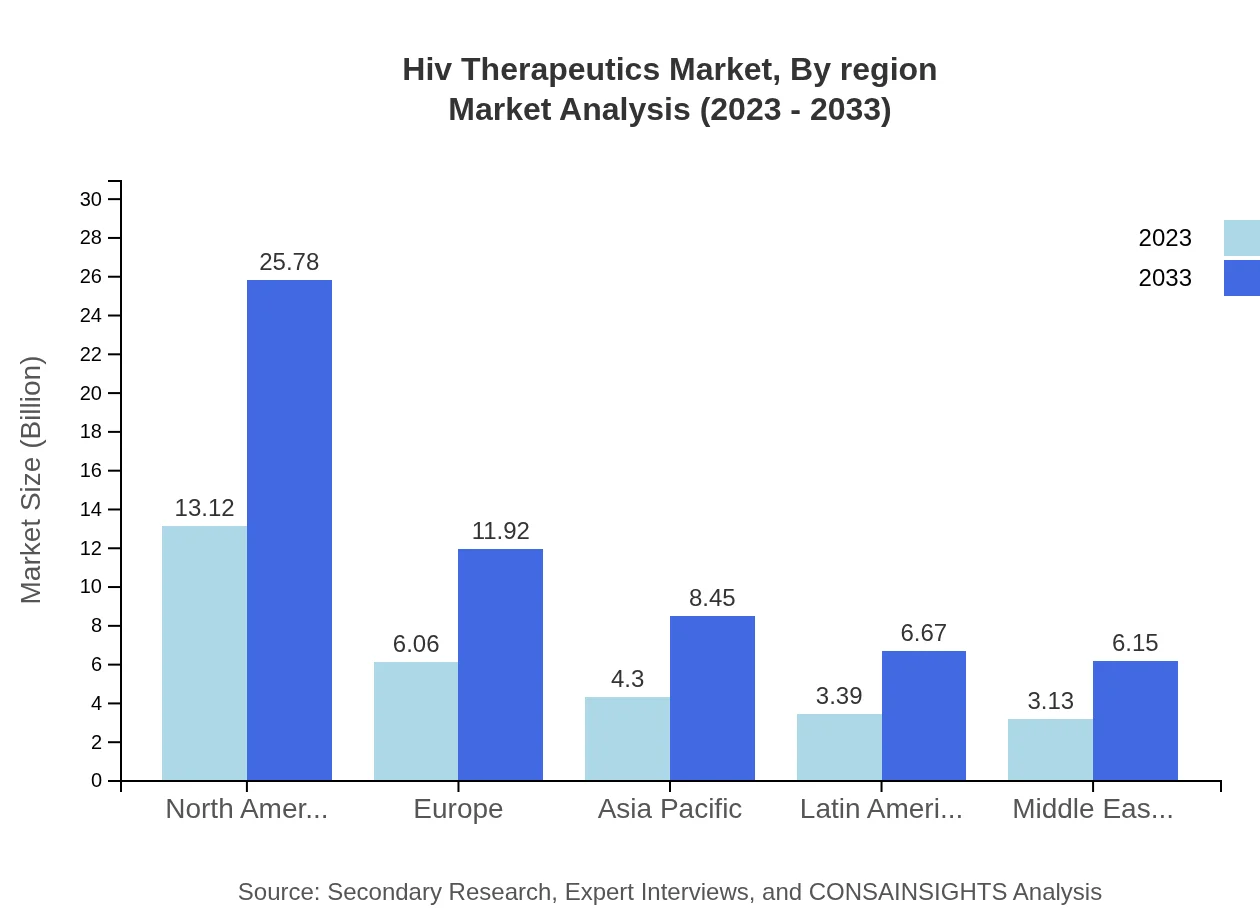
HIV Therapeutics Market Analysis Report by Region
Europe Hiv Therapeutics Market Report:
The European market is expected to grow from USD 8.33 billion in 2023 to USD 16.36 billion by 2033. The focus on patient-centric therapies and continual funding for HIV-related research are significant contributors to this growth.Asia Pacific Hiv Therapeutics Market Report:
The Asia Pacific region holds a substantial share in the HIV therapeutics market, valued at USD 5.78 billion in 2023, and expected to grow to USD 11.36 billion by 2033. The growing prevalence of HIV in countries like India and China and increasing awareness and availability of treatments are key growth factors.North America Hiv Therapeutics Market Report:
North America leads the market with an expected value increase from USD 9.86 billion in 2023 to USD 19.38 billion by 2033. The region experiences a high prevalence of HIV, with advanced healthcare infrastructure and strong research initiatives driving growth.South America Hiv Therapeutics Market Report:
In South America, the HIV therapeutics market is projected to grow from USD 1.92 billion in 2023 to USD 3.77 billion in 2033. Increased governmental support and funding for HIV/AIDS programs are expediting treatment accessibility.Middle East & Africa Hiv Therapeutics Market Report:
In the Middle East and Africa, the market is anticipated to expand from USD 4.11 billion in 2023 to USD 8.08 billion by 2033, driven by increased government initiatives to enhance healthcare access and the provision of antiretroviral treatments.Tell us your focus area and get a customized research report.
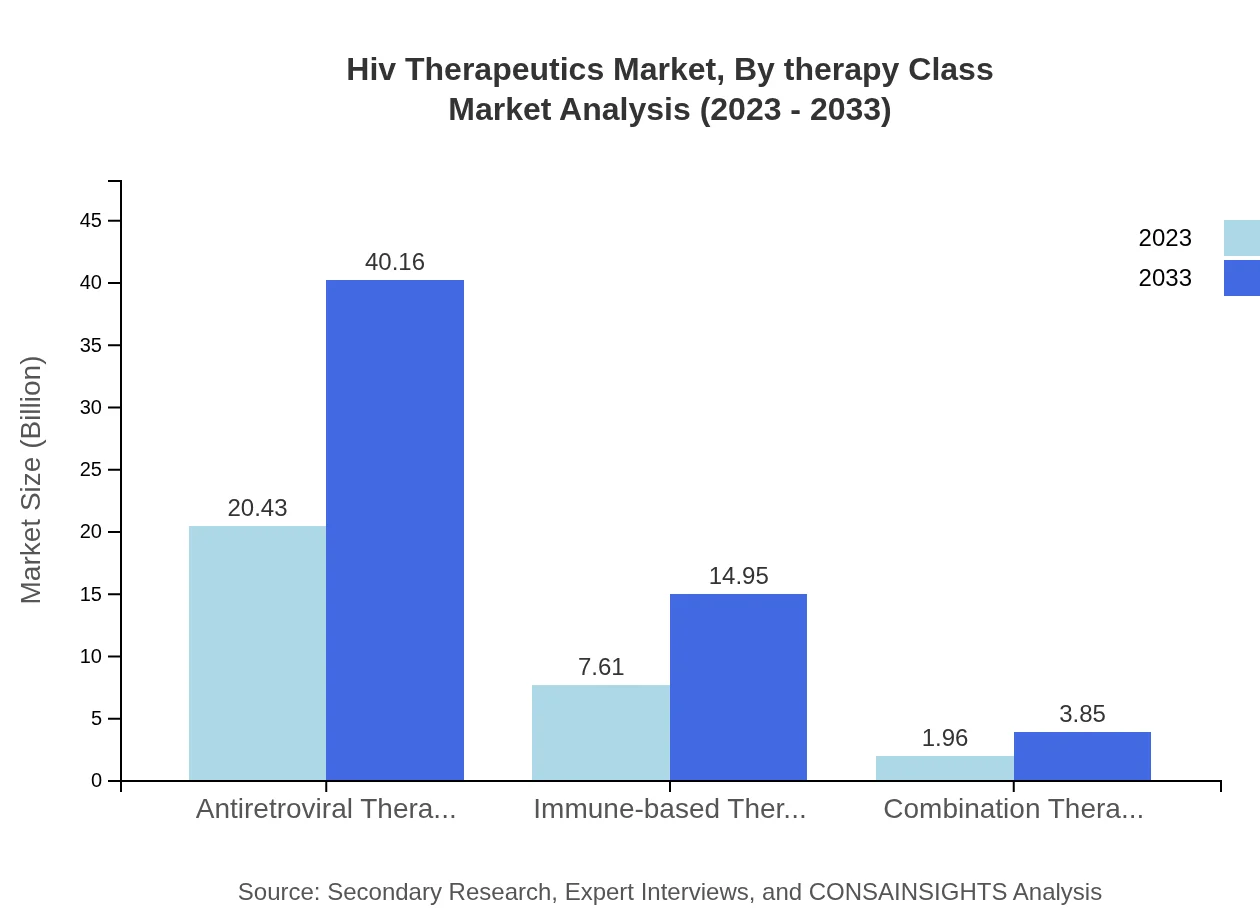
Hiv Therapeutics Market Analysis By Therapy Class
Within the HIV therapeutics market, antiretroviral therapies dominate, making up over 68% of the market share in 2023. Innovative options like immune-based therapies and combination therapies are also gaining traction among healthcare providers for their enhanced efficacy.
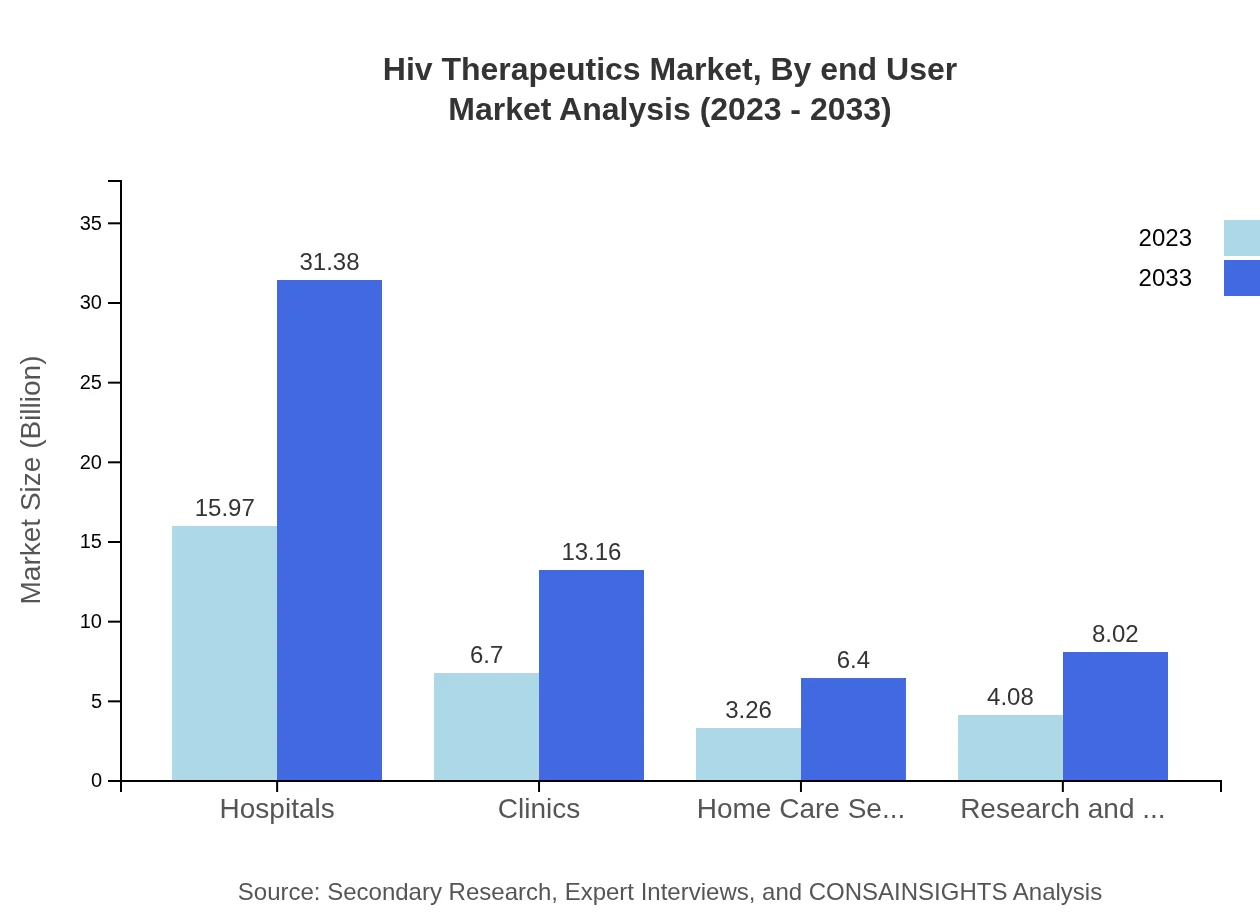
Hiv Therapeutics Market Analysis By End User
The primary end-users of HIV therapeutics include hospitals, clinics, home care settings, and research institutions. In 2023, hospitals account for a significant market share, attributed to the extensive patient base and comprehensive treatment services offered.
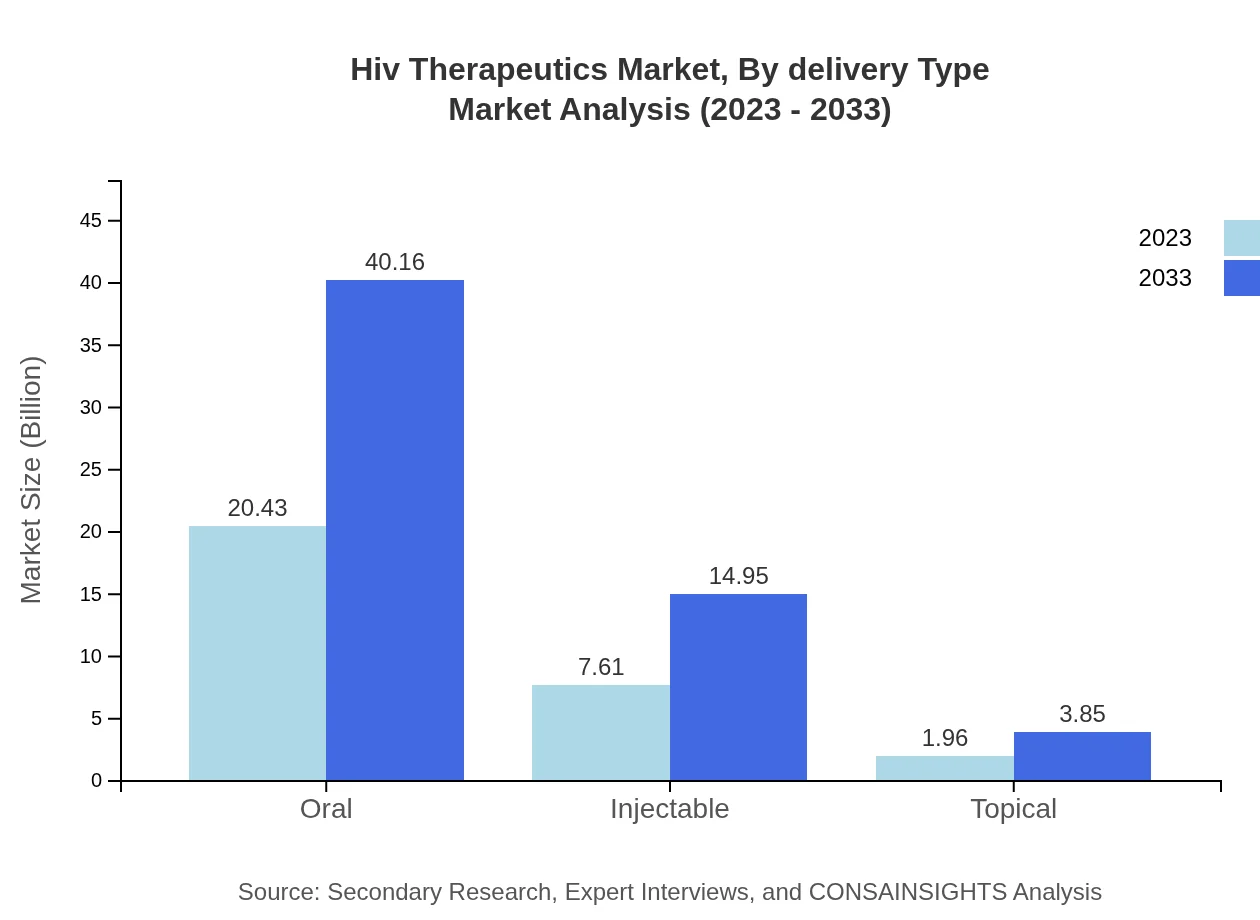
Hiv Therapeutics Market Analysis By Delivery Type
In terms of delivery types, oral formulations dominate the market with an impressive share of 68.11% due to ease of use and patient compliance. Injectable options are also prevalent, growing steadily as patient-centered treatment approaches are adopted.
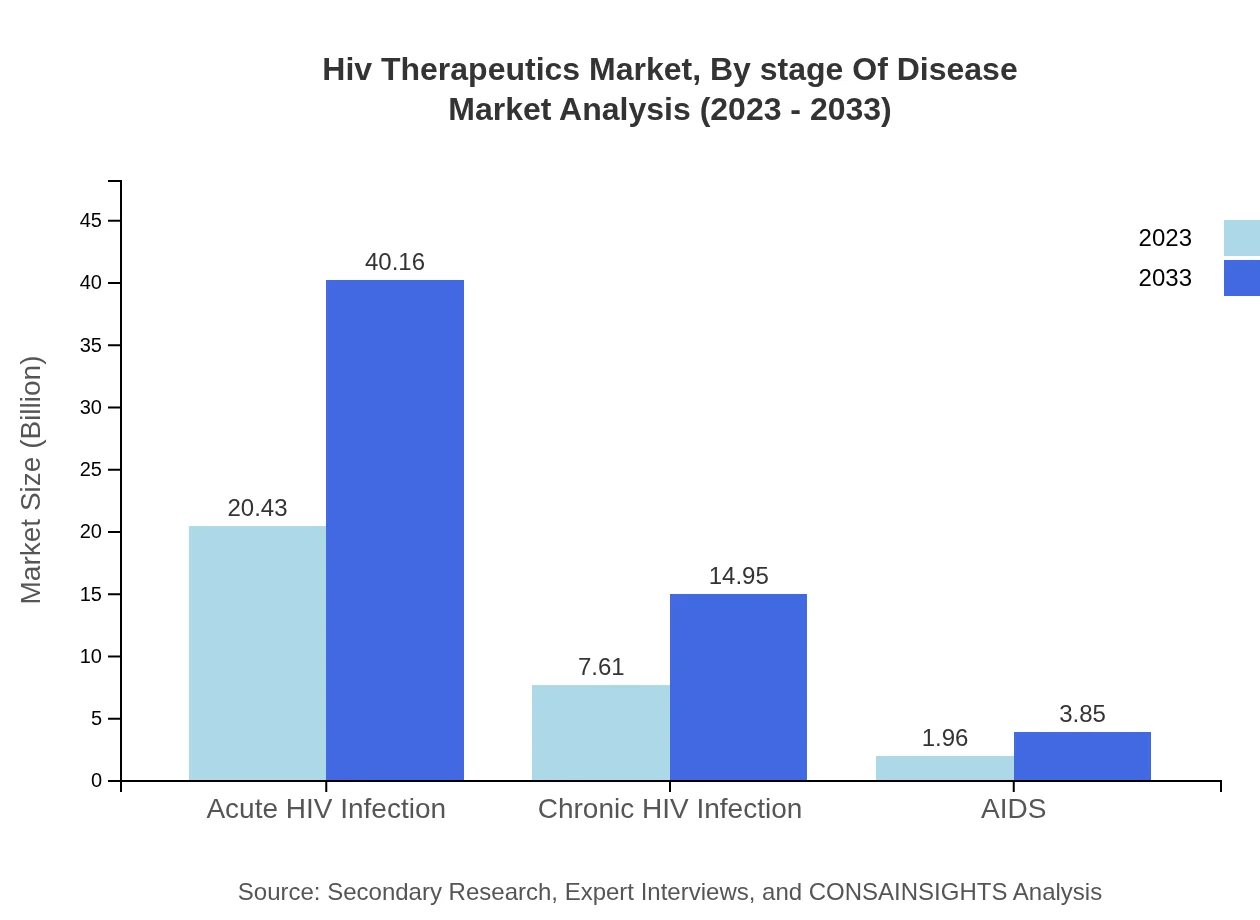
Hiv Therapeutics Market Analysis By Stage Of Disease
The market is segmented by stage of disease, primarily focusing on acute and chronic HIV infections. Acute infections represent a significant share, indicating the need for urgent treatment regimens that effectively manage the disease's progression.
Hiv Therapeutics Market Analysis By Region
Regional analysis shows North America and Europe leading in market size, driven by high treatment accessibility and innovative healthcare solutions. However, regions like Asia Pacific and the Middle East and Africa exhibit the highest growth potential due to increasing diagnosis rates and government support for HIV initiatives.
HIV Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in HIV Therapeutics Industry
Gilead Sciences:
Gilead Sciences is a leading biopharmaceutical company known for its innovative HIV therapies, including Truvada and Biktarvy, significantly contributing to the global efforts against HIV.Viiv Healthcare:
Viiv Healthcare specializes in the development of HIV treatments, providing a range of effective medications that improve the quality of life for patients and support adherence to treatment.Johnson & Johnson:
Johnson & Johnson develops notable HIV therapeutic options including the first long-acting injectable treatment, thereby significantly influencing the treatment landscape.Merck & Co.:
Merck & Co. is recognized for its robust pipeline in HIV therapies, focusing on pioneering antiviral medications with improved efficacy and safety profiles.We're grateful to work with incredible clients.









FAQs
What is the market size of HIV therapeutics?
The global HIV therapeutics market is projected to reach approximately $30 billion by 2033, growing at a CAGR of 6.8% from 2023. This growth is driven by advancements in treatment options and increasing awareness of HIV management across diverse populations.
What are the key market players or companies in the HIV therapeutics industry?
Leading companies in the HIV therapeutics market include Gilead Sciences, Johnson & Johnson, Merck & Co., and AbbVie. These firms drive innovation and generate growth through extensive research, development, and partnerships within the pharmaceutical sector.
What are the primary factors driving the growth in the HIV therapeutics industry?
Key growth drivers include improved healthcare access in developing regions, advancements in antiretroviral therapies, and increased government funding for HIV research and treatment initiatives. Additionally, greater awareness of HIV prevention and treatment contributes significantly to market expansion.
Which region is the fastest Growing in the HIV therapeutics market?
The Asia Pacific region is projected to witness significant growth in the HIV therapeutics market, with a market size expected to rise from $5.78 billion in 2023 to $11.36 billion by 2033, driven by rising healthcare investments and improving access to treatment.
Does ConsaInsights provide customized market report data for the HIV therapeutics industry?
Yes, ConsaInsights offers customized market reports for the HIV therapeutics industry, addressing specific client needs and providing tailored insights that enhance strategic decision-making and investment planning.
What deliverables can I expect from this HIV therapeutics market research project?
Clients can expect comprehensive deliverables including detailed market analyses, forecasts, competitive landscape assessments, trend analyses, and insights on regional markets, helping to inform strategic business planning and investment opportunities.
What are the market trends of HIV therapeutics?
Recent trends in the HIV therapeutics market include the rise of long-acting injectable therapies, combination therapies for better patient adherence, and a growing focus on personalized medicine, which aims to tailor treatment based on individual patient needs.