Hpv Testing And Pap Test Market Report
Published Date: 31 January 2026 | Report Code: hpv-testing-and-pap-test
Hpv Testing And Pap Test Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the HPV Testing and Pap Test markets, covering critical data and insights, including trends, market size, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
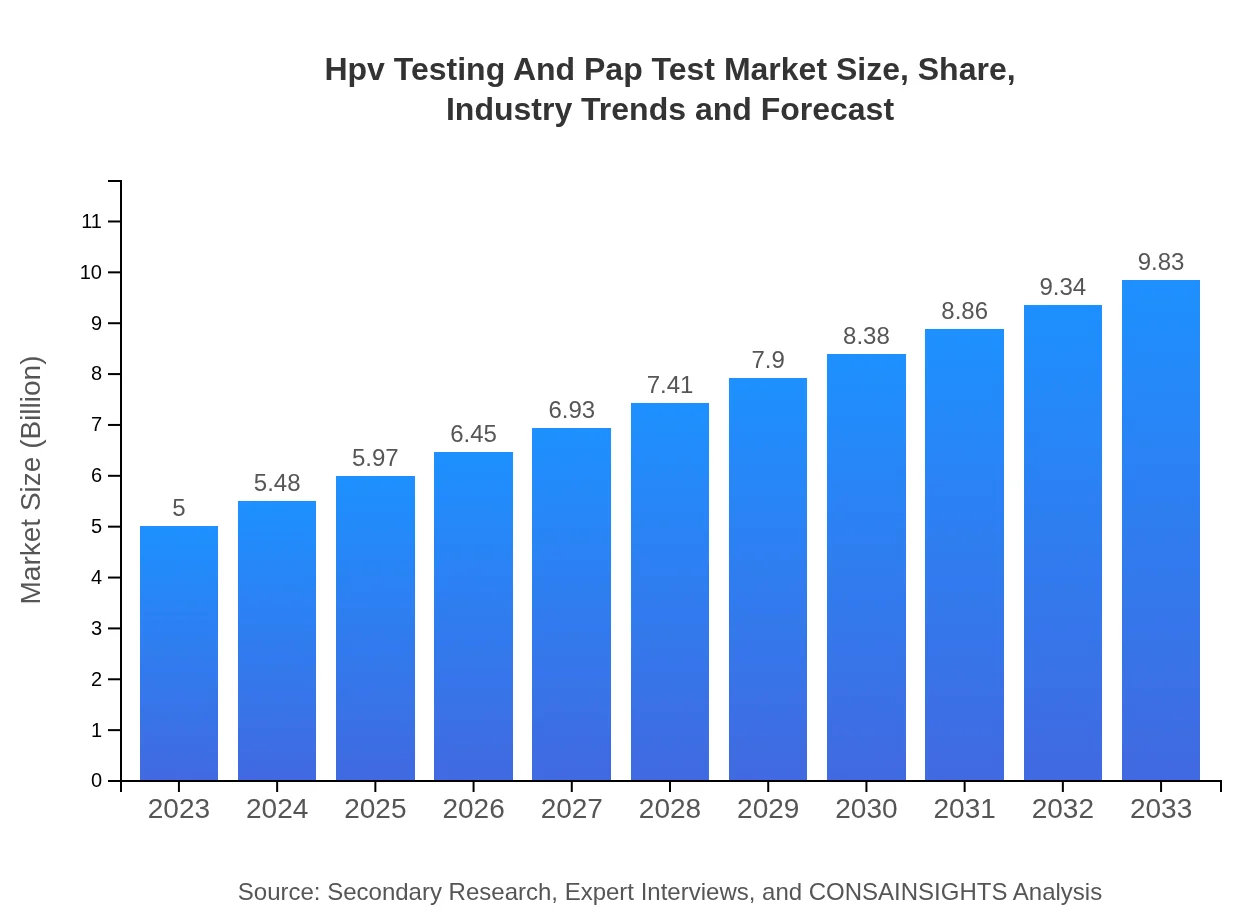
| 2023 Market Size | $5.00 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $9.83 Billion |
| Top Companies | Roche Diagnostics, Hologic, Inc., BD (Becton, Dickinson and Company), Genomic Health |
| Last Modified Date | 31 January 2026 |
Hpv Testing And Pap Test Market Overview
Customize Hpv Testing And Pap Test Market Report market research report
- ✔ Get in-depth analysis of Hpv Testing And Pap Test market size, growth, and forecasts.
- ✔ Understand Hpv Testing And Pap Test's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hpv Testing And Pap Test
What is the Market Size & CAGR of Hpv Testing And Pap Test market in 2023?
Hpv Testing And Pap Test Industry Analysis
Hpv Testing And Pap Test Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hpv Testing And Pap Test Market Analysis Report by Region
Europe Hpv Testing And Pap Test Market Report:
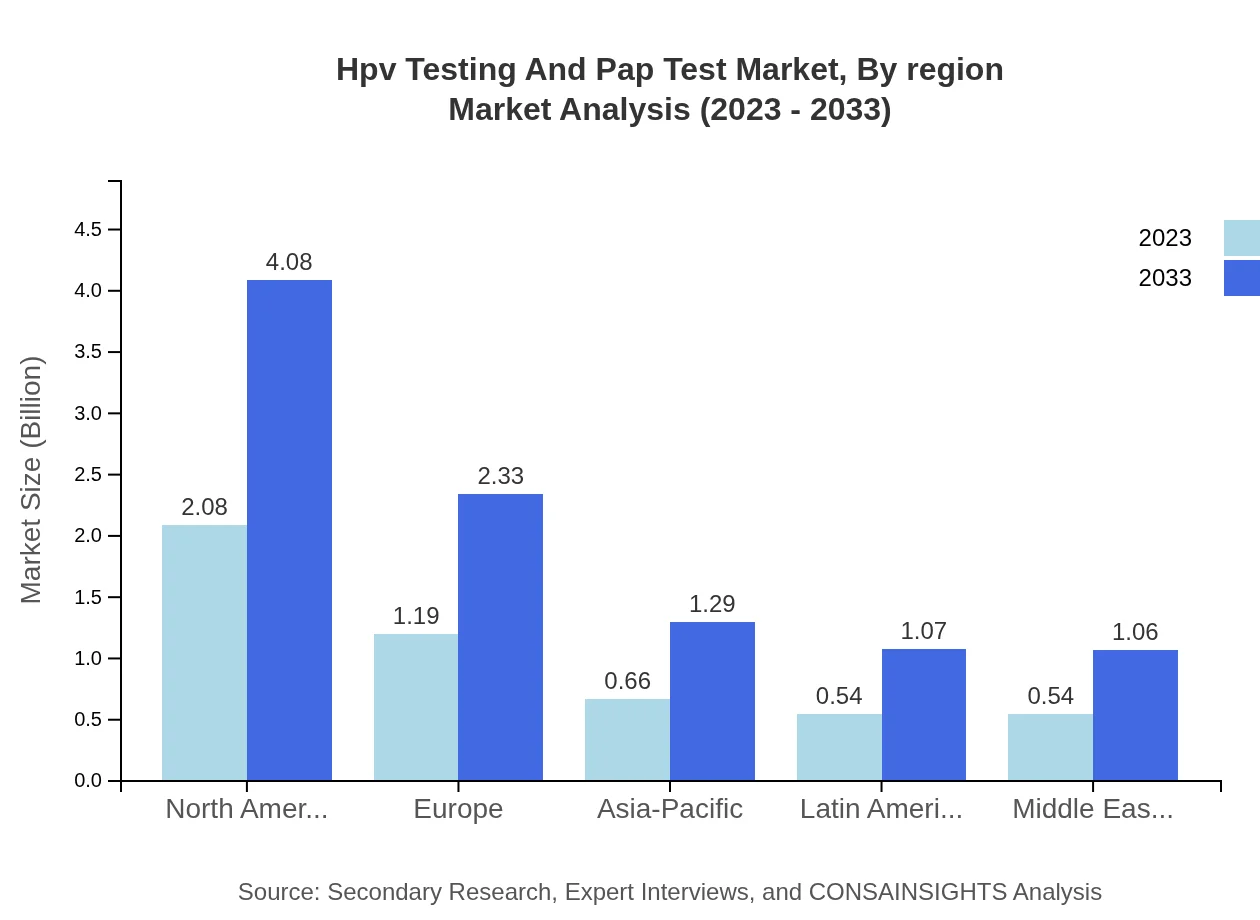
The European market is expected to expand from $1.44 billion in 2023 to $2.83 billion by 2033. Strong government policies on cancer prevention and increasing investment in healthcare infrastructure are crucial factors for this growth.Asia Pacific Hpv Testing And Pap Test Market Report:
The Asia Pacific region is anticipated to witness significant growth, with the market expected to reach $1.87 billion by 2033, increasing from $0.95 billion in 2023. Factors contributing to this growth include rising healthcare awareness and government initiatives to promote cervical cancer screening.North America Hpv Testing And Pap Test Market Report:
North America leads the market, projected to grow from $1.86 billion in 2023 to $3.66 billion in 2033. The region benefits from high healthcare spending, advanced technology adoption, and widespread awareness of HPV-related health issues.South America Hpv Testing And Pap Test Market Report:
In South America, the HPV Testing and Pap Test market is set to grow from $0.43 billion in 2023 to $0.84 billion by 2033, attributed to increasing public health efforts and access to advanced medical technologies.Middle East & Africa Hpv Testing And Pap Test Market Report:
The Middle East and Africa market is also growing, expected to rise from $0.32 billion in 2023 to $0.63 billion by 2033. Ongoing health awareness campaigns and improved access to testing are driving market gains.Tell us your focus area and get a customized research report.
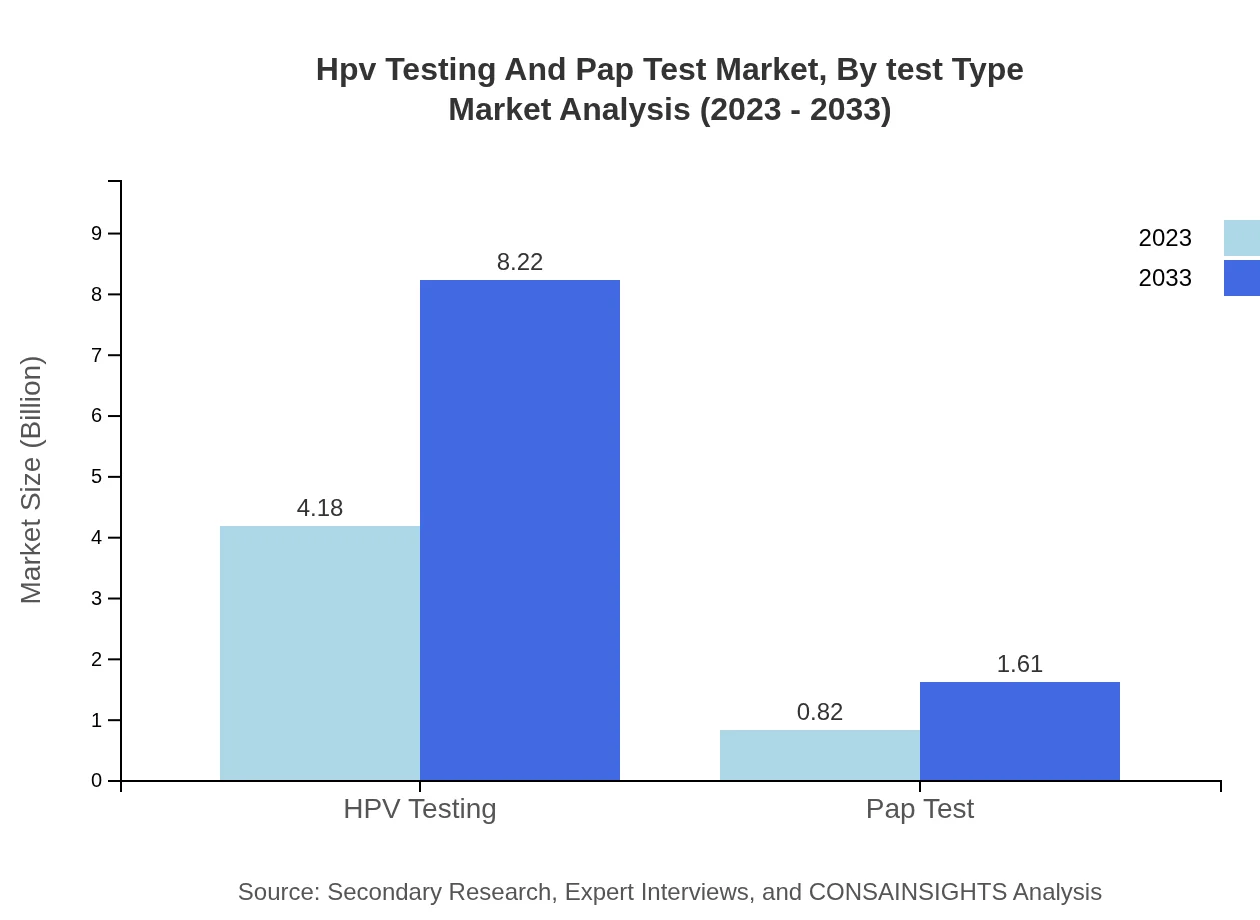
Hpv Testing And Pap Test Market Analysis By Test Type
The HPV Testing segment holds the majority share of the market, accounting for $4.18 billion in 2023 and expected to grow to $8.22 billion by 2033, representing 83.6% of the overall market. In contrast, the Pap Test segment stands at $0.82 billion in 2023 and is projected to reach $1.61 billion by 2033, comprising 16.4% of the market share.
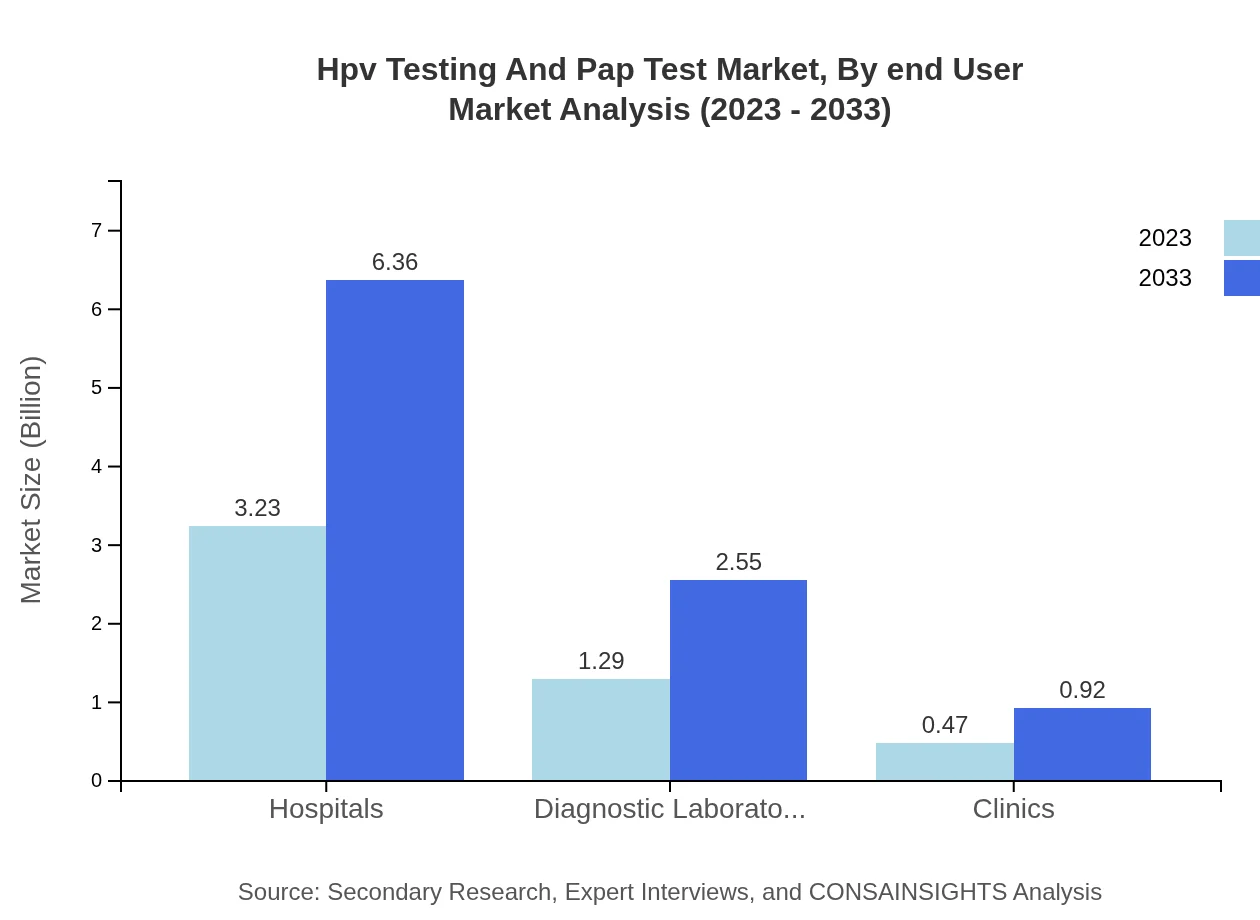
Hpv Testing And Pap Test Market Analysis By End User
The primary end-users of HPV Testing and Pap Test services include hospitals, diagnostic laboratories, and clinics. In 2023, hospitals represented $3.23 billion (64.69% share), while diagnostic laboratories accounted for $1.29 billion (25.9%). Clinics contributed $0.47 billion (9.41%) to the market, indicating a diverse landscape in terms of service provision.
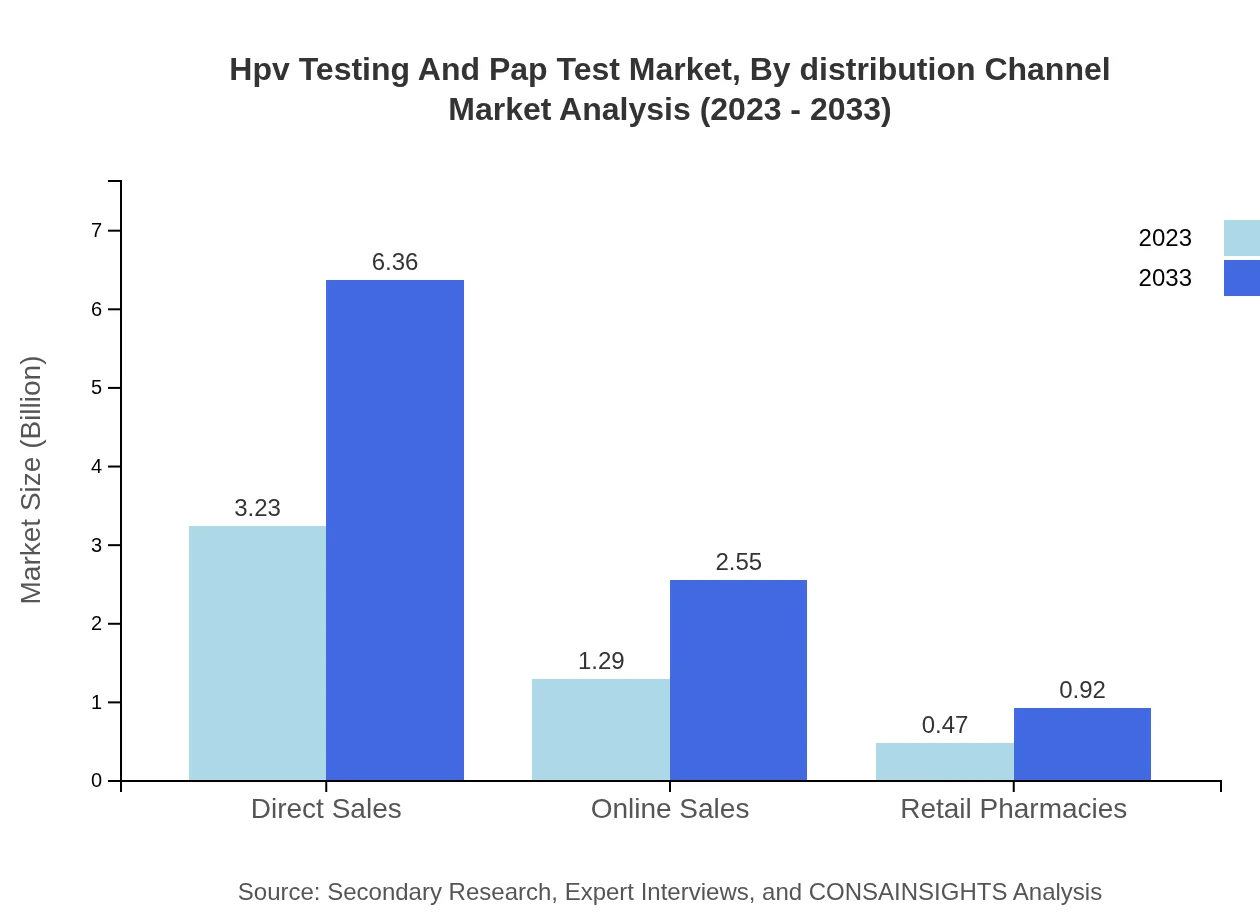
Hpv Testing And Pap Test Market Analysis By Distribution Channel
The distribution channels for HPV Testing and Pap Test services encompass direct sales, online sales, and retail pharmacies. Direct sales dominate with $3.23 billion (64.69% share) in 2023, while online sales contribute $1.29 billion (25.9%), and retail pharmacies account for $0.47 billion (9.41%). This segmentation highlights the different avenues through which these services reach consumers.
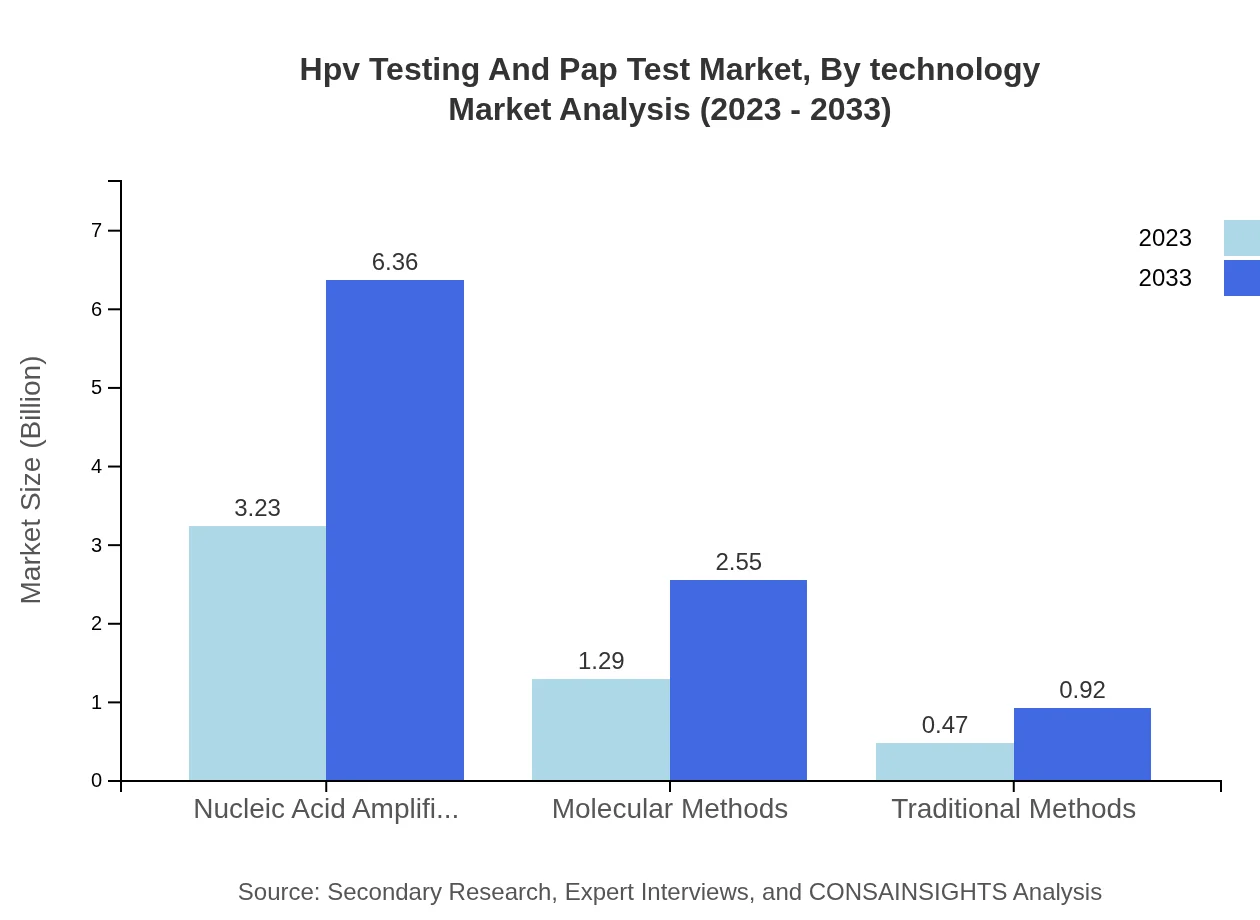
Hpv Testing And Pap Test Market Analysis By Technology
Nucleic Acid Amplification Tests (NAATs) represent the leading technology within the market, valued at $3.23 billion (64.69% share) in 2023, with a projected growth trajectory. Molecular methods and traditional methods follow, with respective shares of 25.9% and 9.41%, indicating evolving preferences for testing methodologies among healthcare providers.
Hpv Testing And Pap Test Market Analysis By Region
Regional analysis indicates North America leads in market size, followed by Europe and the Asia Pacific. North America's market value of $1.86 billion in 2023 (41.55% share) showcases its dominance, whereas Europe and Asia Pacific hold shares of 23.7% and 13.1%, respectively, underscoring the competitive landscape in varied global markets.
Hpv Testing And Pap Test Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hpv Testing And Pap Test Industry
Roche Diagnostics:
Roche is a leader in the development of informative and impactful diagnostic solutions, focusing on cervical cancer prevention and testing innovations.Hologic, Inc.:
Hologic specializes in medical technology focused on women's health, with a suite of products diversifying the HPV testing market.BD (Becton, Dickinson and Company):
BD provides comprehensive solutions that enhance the quality of cervical cancer screening, driving advancements in testing methodologies.Genomic Health:
Genomic Health is at the forefront of molecular diagnostics, significantly impacting the oncology space with its HPV testing methodologies.We're grateful to work with incredible clients.









FAQs
What is the market size of HPV testing and Pap test?
The HPV testing and Pap test market size is projected to reach approximately $5 billion by 2033, growing at a CAGR of 6.8% from 2023. This reflects increasing awareness and advancements in diagnostic technologies.
What are the key market players or companies in the HPV testing and Pap test industry?
Key market players include established diagnostic manufacturers and laboratories that specialize in women’s health, such as Roche, Hologic, and LabCorp, contributing to market innovations and enhanced testing capabilities.
What are the primary factors driving the growth in the HPV testing and Pap test industry?
The growth is driven by rising incidences of cervical cancer, increasing government health initiatives, the push for early detection methods, and continuous advancements in testing technologies that improve accuracy and patient convenience.
Which region is the fastest Growing in the HPV testing and Pap test market?
North America is the fastest-growing region, with the market expected to increase from $1.86 billion in 2023 to $3.66 billion by 2033, driven by high adoption rates of advanced screening technologies.
Does ConsaInsights provide customized market report data for the HPV testing and Pap test industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs, enabling stakeholders to gain insights that are directly applicable to their unique market challenges and opportunities.
What deliverables can I expect from this HPV testing and Pap test market research project?
Deliverables include comprehensive market analysis, trend evaluation, competitive landscape assessments, and forecasting of growth trajectories, helping clients make informed strategic decisions.
What are the market trends of HPV testing and Pap test?
Current trends include increasing reliance on HPV testing over traditional Pap tests, integration of molecular methods, and expansion of telehealth services, enhancing access and convenience for patients.