Human Embryonic Stem Cells Market Report
Published Date: 31 January 2026 | Report Code: human-embryonic-stem-cells
Human Embryonic Stem Cells Market Size, Share, Industry Trends and Forecast to 2033
This report provides an insightful analysis of the Human Embryonic Stem Cells market, examining key trends, growth projections, and competitive landscapes from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
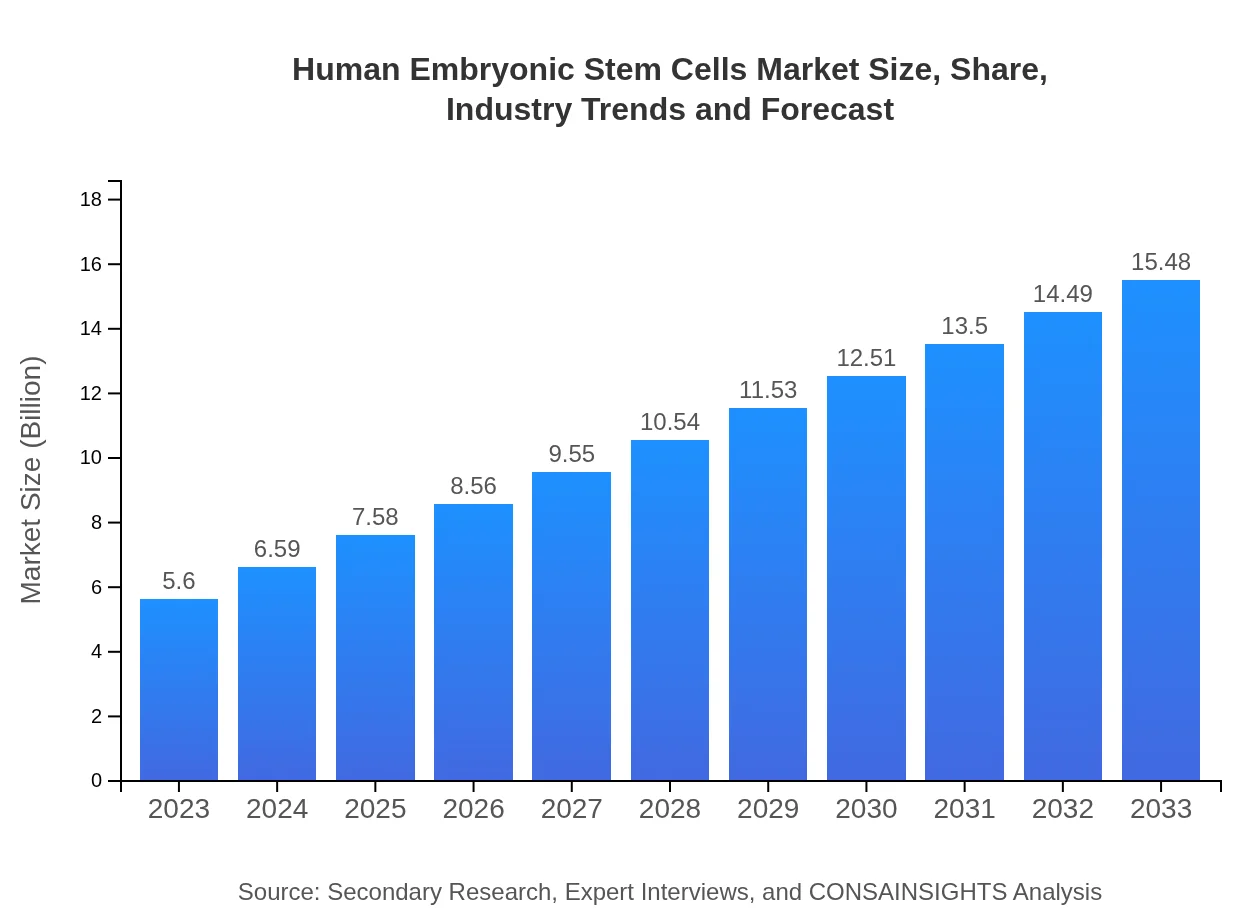
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 10.3% |
| 2033 Market Size | $15.48 Billion |
| Top Companies | Thermo Fisher Scientific, Merck KGaA, Lonza Group, StemCells, Inc. |
| Last Modified Date | 31 January 2026 |
Human Embryonic Stem Cells Market Overview
Customize Human Embryonic Stem Cells Market Report market research report
- ✔ Get in-depth analysis of Human Embryonic Stem Cells market size, growth, and forecasts.
- ✔ Understand Human Embryonic Stem Cells's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Human Embryonic Stem Cells
What is the Market Size & CAGR of Human Embryonic Stem Cells market in 2023?
Human Embryonic Stem Cells Industry Analysis
Human Embryonic Stem Cells Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Human Embryonic Stem Cells Market Analysis Report by Region
Europe Human Embryonic Stem Cells Market Report:
In Europe, the HESC market is valued at approximately $1.73 billion in 2023 and is anticipated to grow to $4.79 billion by 2033. This growth can be attributed to extensive research activities and a strong focus on developing ethical stem cell therapies.Asia Pacific Human Embryonic Stem Cells Market Report:
In the Asia Pacific, the HESC market has seen significant growth, reaching approximately $1.06 billion in 2023 and projected to grow to $2.93 billion by 2033. This region benefits from increasing investments in biotechnology and regenerative medicine, with countries like China and Japan at the forefront of stem cell research.North America Human Embryonic Stem Cells Market Report:
North America represents one of the largest markets for HESCs, valued at $1.98 billion in 2023, projected to reach $5.48 billion by 2033. The U.S. maintains a robust infrastructure for biotechnological research, supported by significant federal funding for stem cell research, making it a leader in the industry.South America Human Embryonic Stem Cells Market Report:
The South American HESC market currently stands at $0.48 billion in 2023 with a forecasted growth to $1.31 billion by 2033. Despite challenges in funding and regulation, the region is gradually developing its research capacities and establishing partnerships to enhance HESC applications.Middle East & Africa Human Embryonic Stem Cells Market Report:
The Middle East and Africa HESC market, while smaller, is seeing growth from a market value of $0.35 billion in 2023 to an estimated $0.97 billion by 2033. Increased collaboration with international research entities and funding opportunities are helping develop this nascent market.Tell us your focus area and get a customized research report.
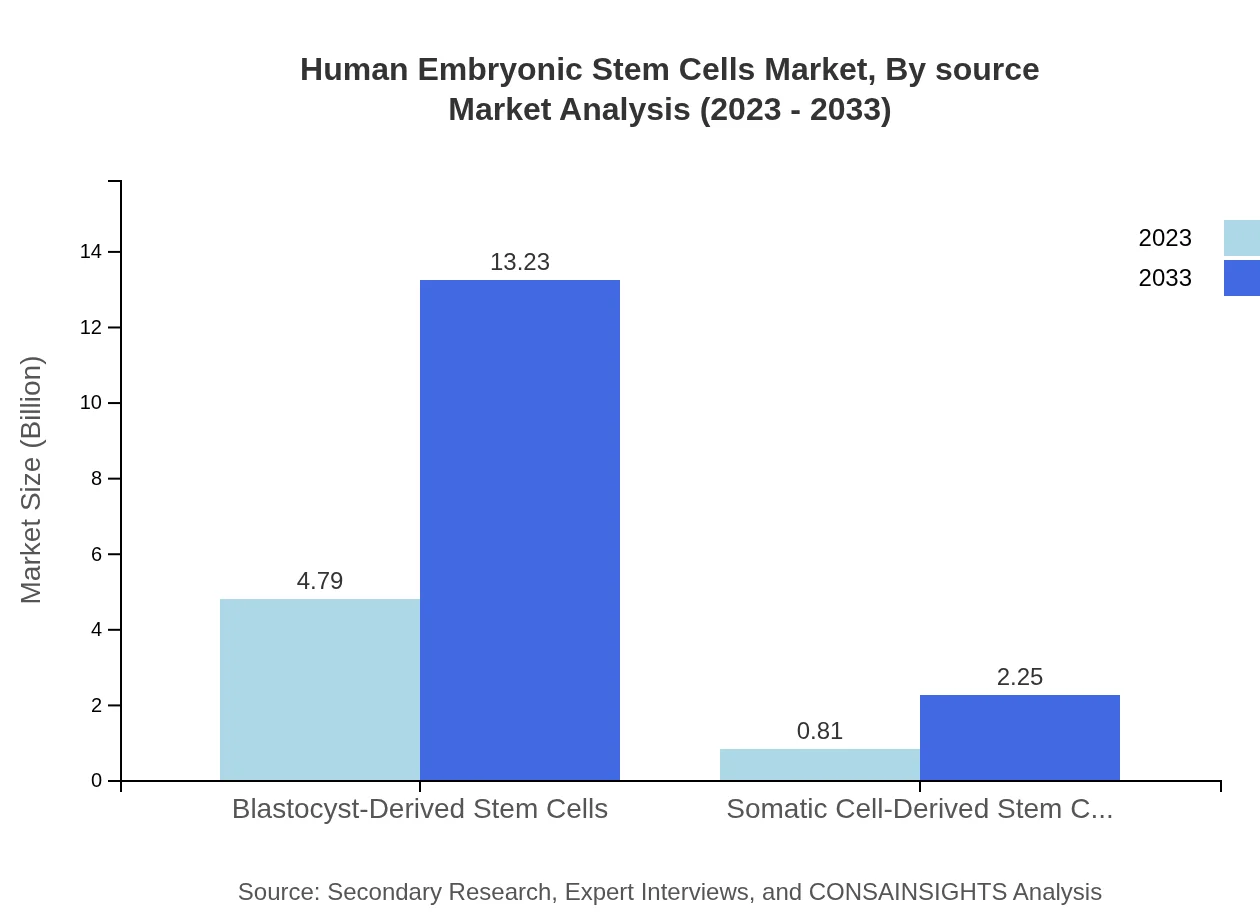
Human Embryonic Stem Cells Market Analysis By Source
The market is predominantly led by blastocyst-derived stem cells, accounting for approximately $4.79 billion in 2023, and anticipated to grow to $13.23 billion by 2033. In contrast, somatic cell-derived stem cells currently represent a market value of $0.81 billion, with expectations of reaching $2.25 billion within the same timeframe.
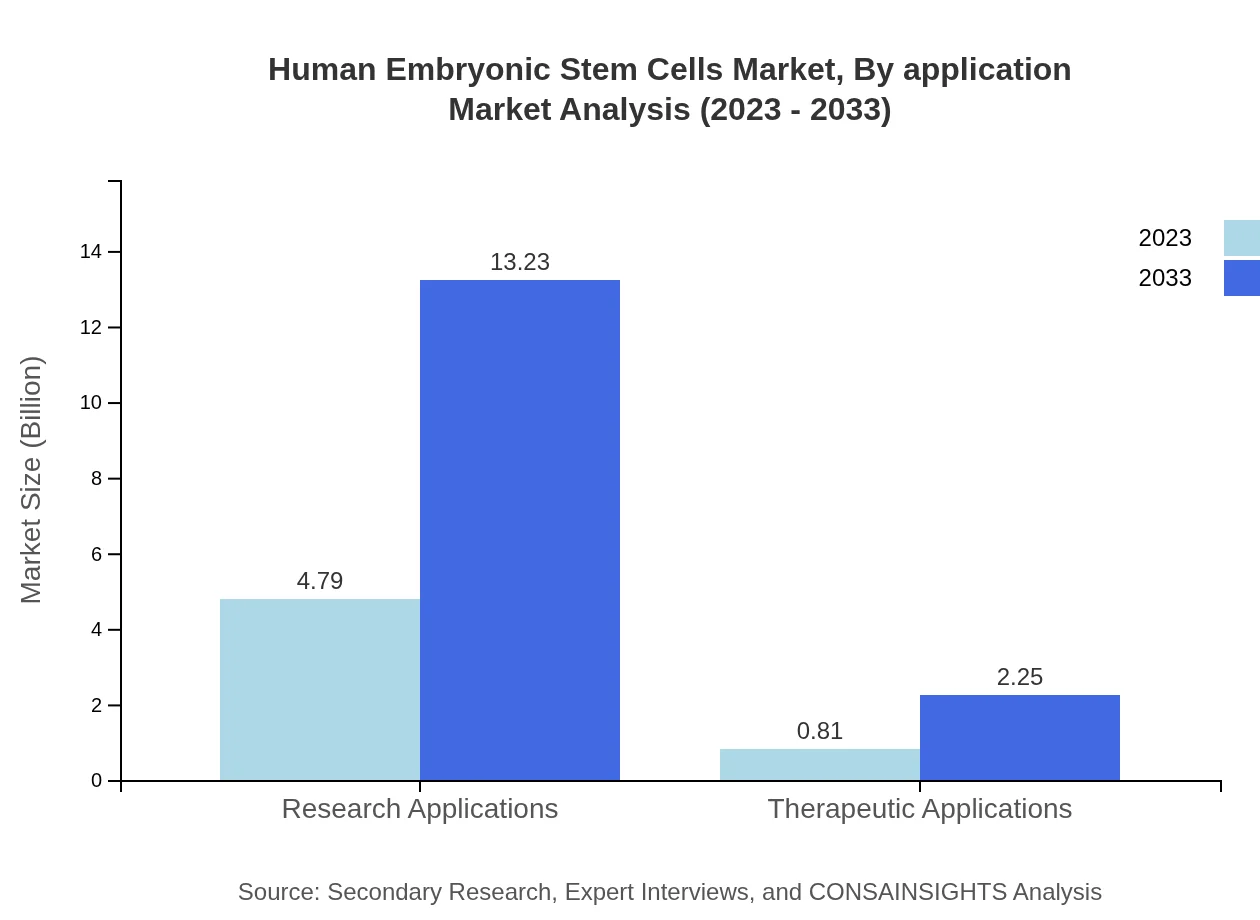
Human Embryonic Stem Cells Market Analysis By Application
The predominant application area for HESCs is research applications, which accounted for a market size of $4.79 billion in 2023 and is projected to expand to $13.23 billion by 2033. Therapeutic applications, while smaller, are growing steadily, with a market size of $0.81 billion in 2023 expected to increase to $2.25 billion over the forecast period.
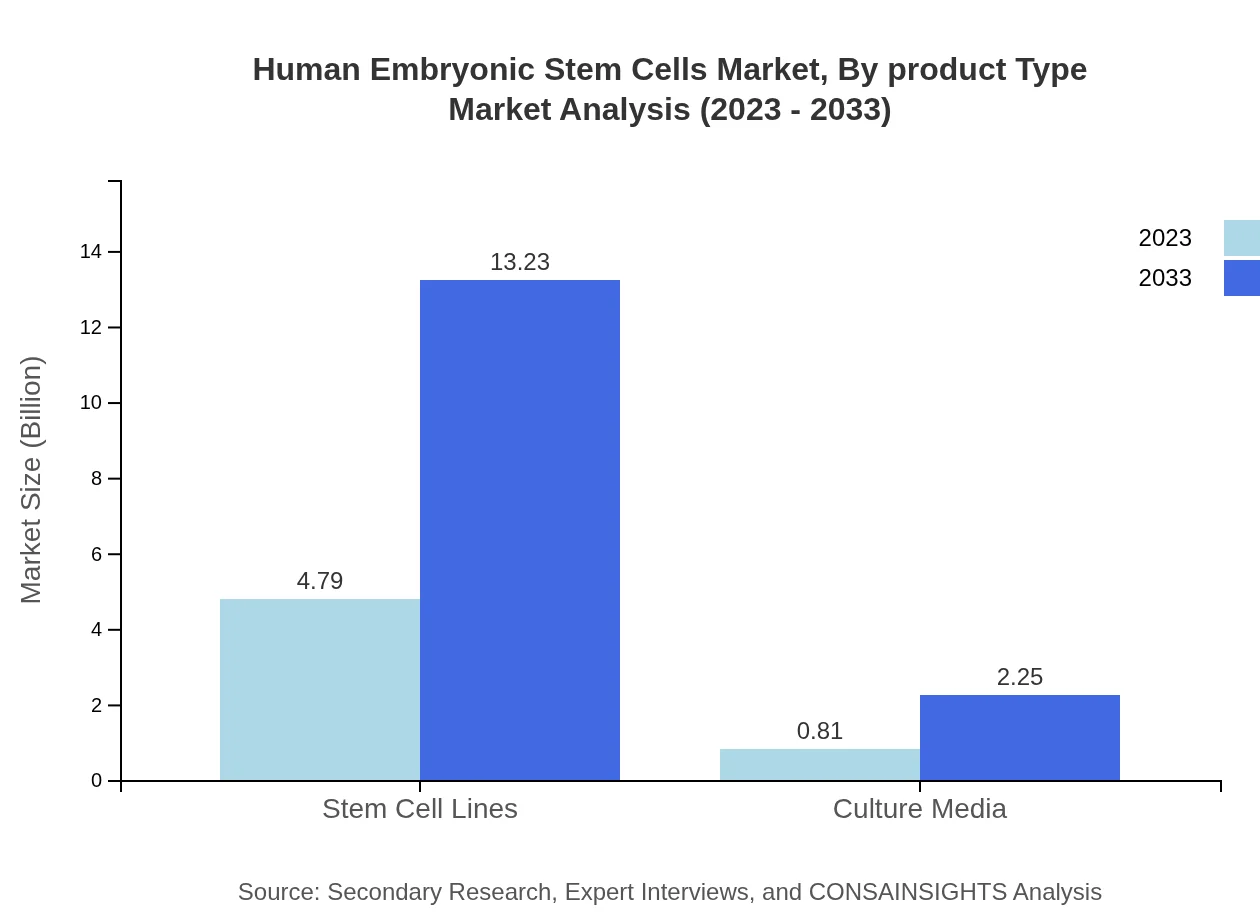
Human Embryonic Stem Cells Market Analysis By Product Type
The HESC market comprises key product types including stem cell lines, valued at $4.79 billion in 2023 and $13.23 billion by 2033, and culture media, which represents a smaller segment with a size of $0.81 billion in 2023 growing to $2.25 billion by 2033.
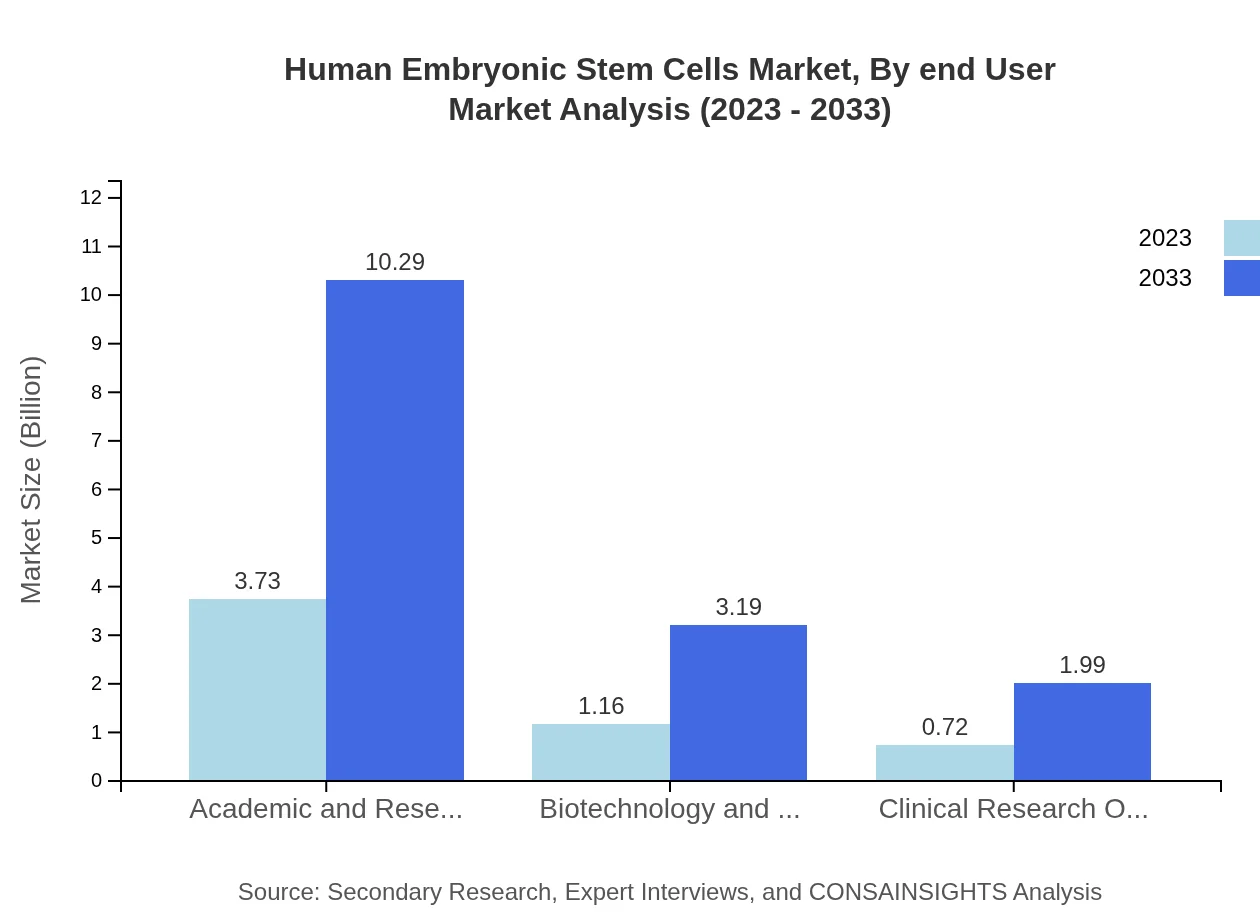
Human Embryonic Stem Cells Market Analysis By End User
The industry encompasses several end-users, including academic and research institutes, which accounted for $3.73 billion in 2023 and are projected to grow to $10.29 billion by 2033. Biotechnology and pharmaceutical companies also represent significant demand, with a market size of $1.16 billion increasing to $3.19 billion by the forecast's end.
Human Embryonic Stem Cells Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Human Embryonic Stem Cells Industry
Thermo Fisher Scientific:
A leader in serving science, Thermo Fisher offers a variety of stem cell research products and services, enhancing the capabilities of many researchers in this field.Merck KGaA:
Merck KGaA plays a prominent role in the HESC market with its innovative range of products that support cell biology and stem cell culture.Lonza Group:
Lonza is known for its high-quality stem cell technologies and services, helping to advance stem cell research and clinical therapies.StemCells, Inc.:
A biotechnology company focused on stem cell research and development, StemCells, Inc. aims to bring innovative therapies to the market.We're grateful to work with incredible clients.









FAQs
What is the market size of human Embryonic Stem Cells?
The human embryonic stem cells market is currently valued at approximately $5.6 billion, with a projected CAGR of 10.3% from 2023 to 2033, indicating robust growth driven by advancements in research and therapeutic applications.
What are the key market players or companies in this human Embryonic Stem Cells industry?
Key players in the human embryonic stem cells market include leading biotechnology and pharmaceutical companies, academic and research institutes, and clinical research organizations, all contributing to innovation and expansion in stem cell research.
What are the primary factors driving the growth in the human Embryonic Stem Cells industry?
The primary factors driving growth include increasing investments in stem cell research, advancements in regenerative medicine, rising applications in drug discovery, and the growing awareness about the potential of stem cells in treating various diseases.
Which region is the fastest Growing in the human Embryonic Stem Cells?
Asia Pacific is currently the fastest-growing region in the human embryonic stem cells market, with its market size expected to grow from $1.06 billion in 2023 to $2.93 billion by 2033, reflecting significant expansion in regional research initiatives.
Does ConsaInsights provide customized market report data for the human Embryonic Stem Cells industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the human embryonic stem cells industry, ensuring relevant insights that align with strategic business goals.
What deliverables can I expect from this human Embryonic Stem Cells market research project?
Deliverables for the human embryonic stem cells market research project include comprehensive reports, detailed market analysis, segmentation data, competitive landscape, and forecasts, helping stakeholders make informed decisions.
What are the market trends of human Embryonic Stem Cells?
Market trends in the human embryonic stem cells sector highlight a surge in research applications, a focus on therapeutic uses, and a shift towards innovative stem cell therapies, reflecting the sector's dynamic evolution.