Humira Market Report
Published Date: 31 January 2026 | Report Code: humira
Humira Market Size, Share, Industry Trends and Forecast to 2033
This report presents an in-depth analysis of the Humira market, exploring critical insights, trends, and data from 2023 to 2033. It includes forecasts on market size and growth, regional analysis, and competitive landscape details vital for stakeholders in the pharmaceutical industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
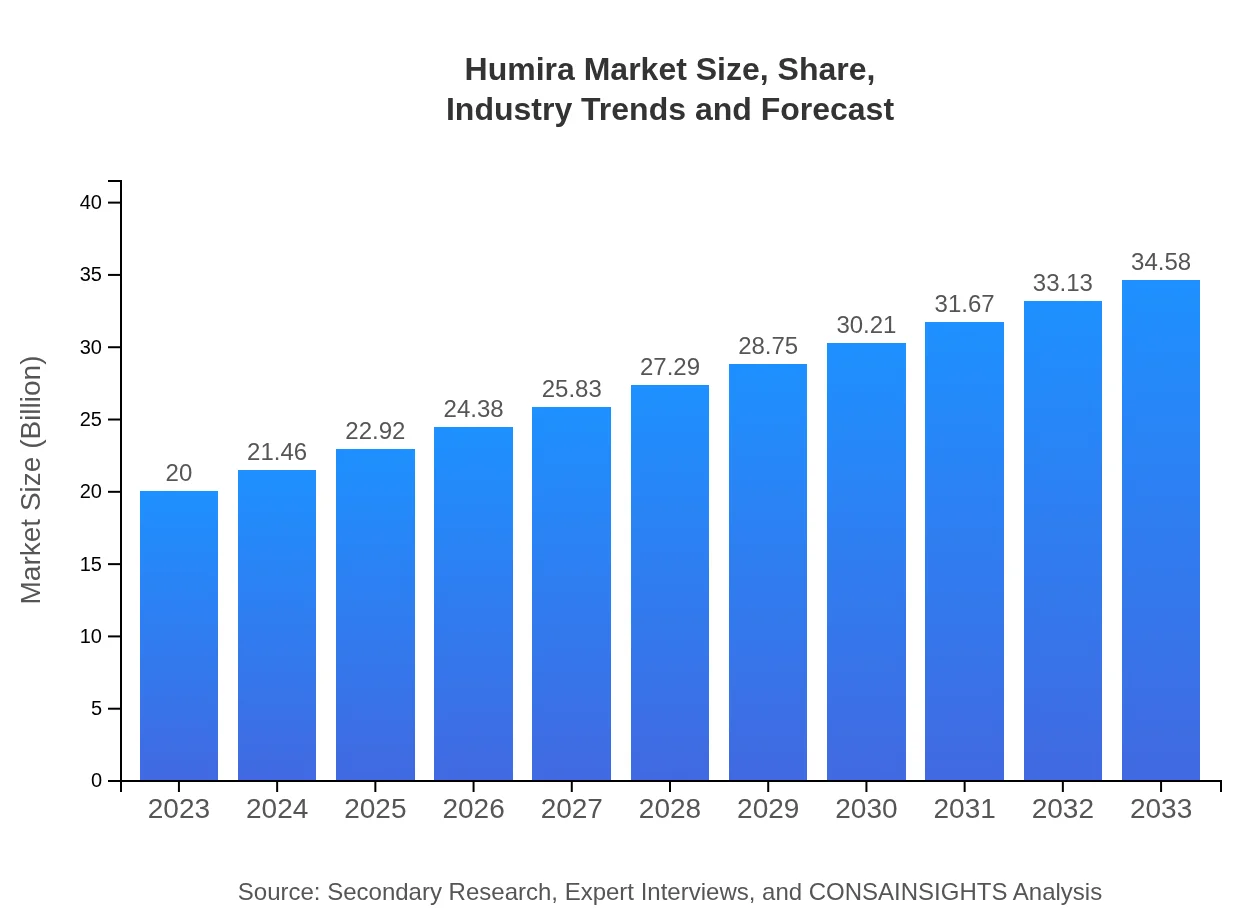
| 2023 Market Size | $20.00 Billion |
| CAGR (2023-2033) | 5.5% |
| 2033 Market Size | $34.58 Billion |
| Top Companies | AbbVie, Amgen, Sandoz, Pfizer |
| Last Modified Date | 31 January 2026 |
Humira Market Overview
Customize Humira Market Report market research report
- ✔ Get in-depth analysis of Humira market size, growth, and forecasts.
- ✔ Understand Humira's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Humira
What is the Market Size & CAGR of Humira market in 2023?
Humira Industry Analysis
Humira Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Humira Market Analysis Report by Region
Europe Humira Market Report:
In Europe, the market is expected to grow from $5.55 billion in 2023 to $9.59 billion by 2033, driven by increasing healthcare investments and innovation in biopharmaceuticals. Regulatory harmonization efforts and the influx of biosimilars will also play roles in shaping competition and pricing strategies.Asia Pacific Humira Market Report:
The Asia Pacific region is projected to witness growth from $3.96 billion in 2023 to $6.85 billion by 2033. Factors driving this growth include increasing healthcare expenditure, improved access to medications, and a rise in the prevalence of autoimmune conditions. Additionally, local partnerships and government initiatives to enhance healthcare systems contribute to this expanding market.North America Humira Market Report:
North America holds the largest market share, with revenues estimated at $6.76 billion in 2023 and projected to grow to $11.69 billion by 2033. The region's robust market is attributed to high healthcare spending, an extensive patient base, and advanced research and development activities. Biosimilar competition and pricing pressures will be key considerations in future growth.South America Humira Market Report:
In South America, Humira's market is expected to increase from $1.03 billion in 2023 to $1.78 billion by 2033. Growth is fueled by a higher focus on treating chronic diseases, enhanced regulatory frameworks, and rising patient awareness about treatment options. However, the market faces challenges like economic volatility and varying access to healthcare.Middle East & Africa Humira Market Report:
The Middle East and Africa region is anticipated to grow from $2.70 billion in 2023 to $4.67 billion by 2033. Growth prospects in this region are enhanced by improvements in healthcare infrastructure, rising demand for effective treatments, and government initiatives aimed at increasing access to pharmaceuticals.Tell us your focus area and get a customized research report.
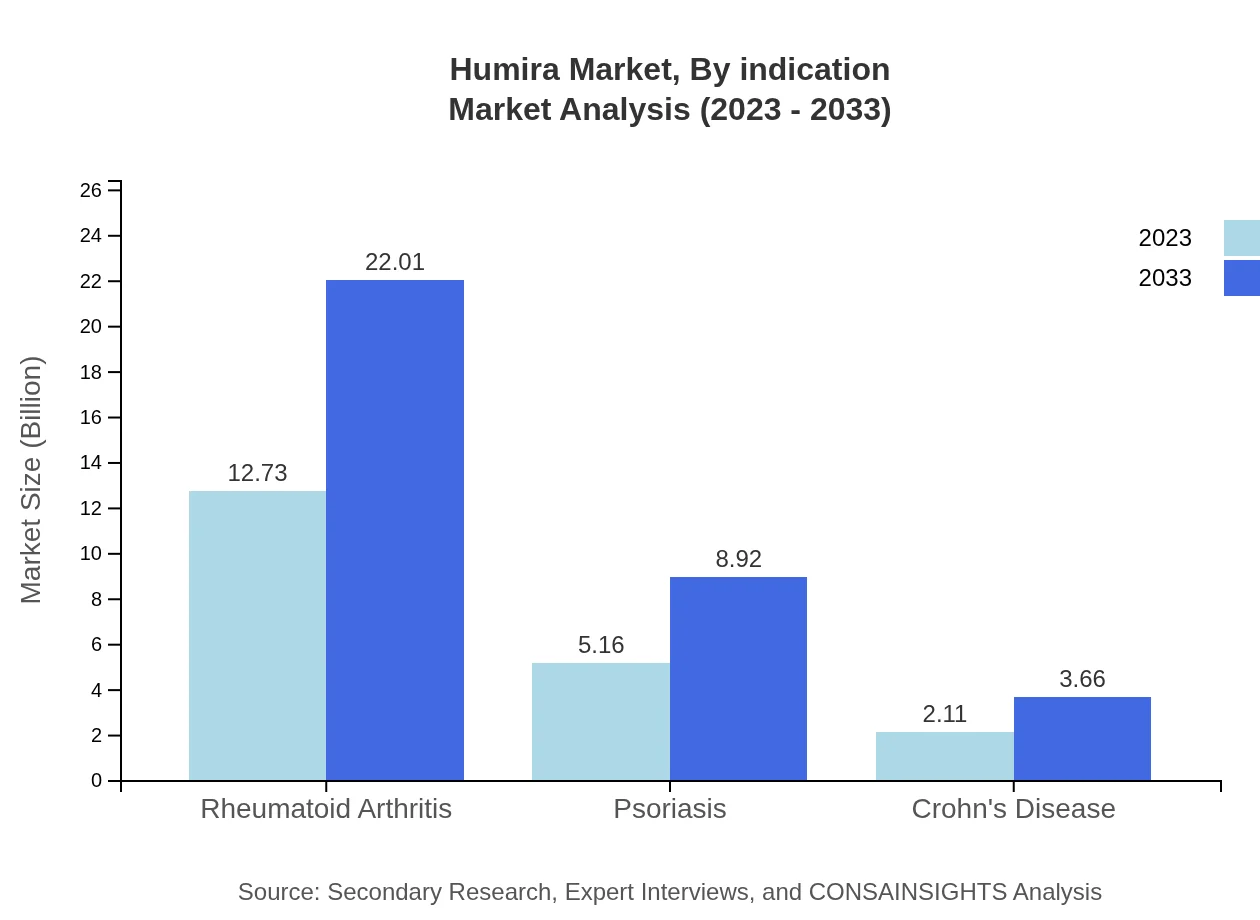
Humira Market Analysis By Indication
In 2023, the market for rheumatoid arthritis stands at $12.73 billion, projected to reach $22.01 billion by 2033, capturing 63.63% of the market share. Psoriasis and Crohn's disease represent segments with respective market sizes of $5.16 billion and $2.11 billion in 2023, expected to grow to $8.92 billion and $3.66 billion by 2033, holding shares of 25.8% and 10.57% respectively.
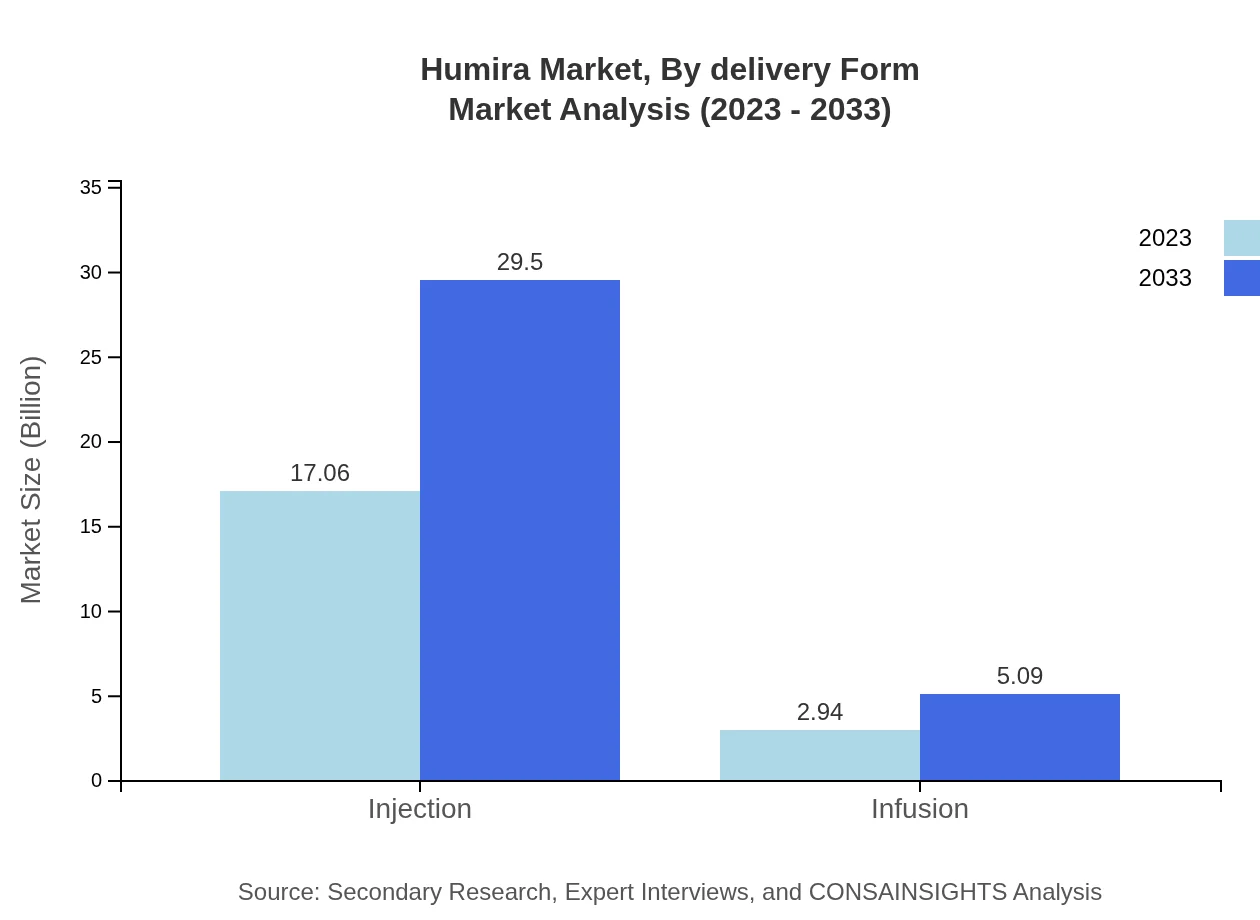
Humira Market Analysis By Delivery Form
The delivery form of Humira includes injections and infusions. In 2023, the injection segment is valued at $17.06 billion, anticipated to rise to $29.50 billion by 2033, capturing 85.29% of the market. The infusion segment, although smaller, will grow from $2.94 billion to $5.09 billion, representing 14.71% of the market.
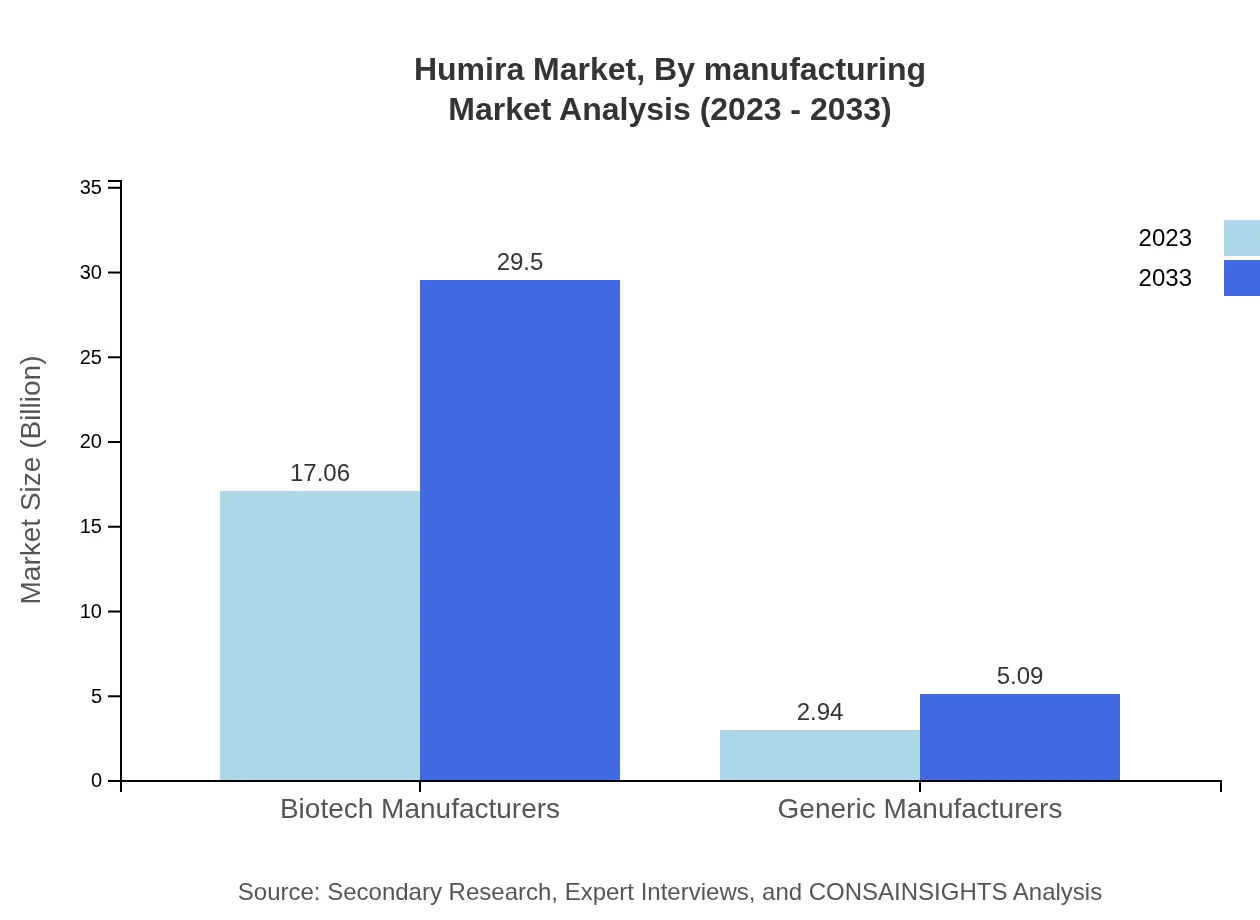
Humira Market Analysis By Manufacturing
Biotech manufacturers hold a significant advantage in the Humira market, generating $17.06 billion in revenue in 2023, projected to grow to $29.50 billion by 2033, with an 85.29% market share. Generic manufacturers, currently valued at $2.94 billion, are expected to achieve $5.09 billion in the same timeframe, representing a 14.71% market share.
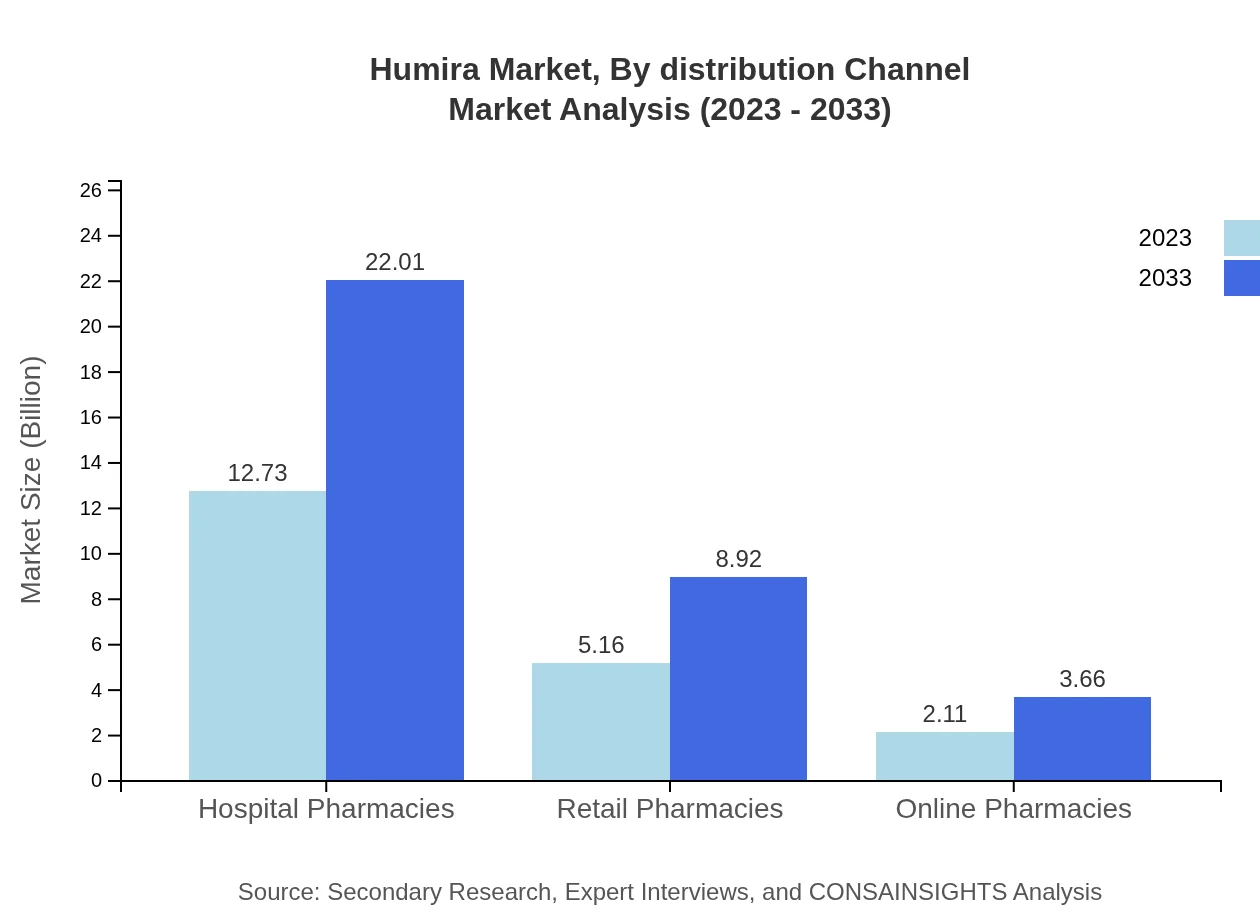
Humira Market Analysis By Distribution Channel
Hospital pharmacies dominate the distribution landscape, with revenues of $12.73 billion in 2023, expected to increase to $22.01 billion by 2033, maintaining a 63.63% share. Retail pharmacies and online pharmacies also feature prominently, with market sizes of $5.16 billion and $2.11 billion, projected to grow to $8.92 billion and $3.66 billion respectively by 2033.
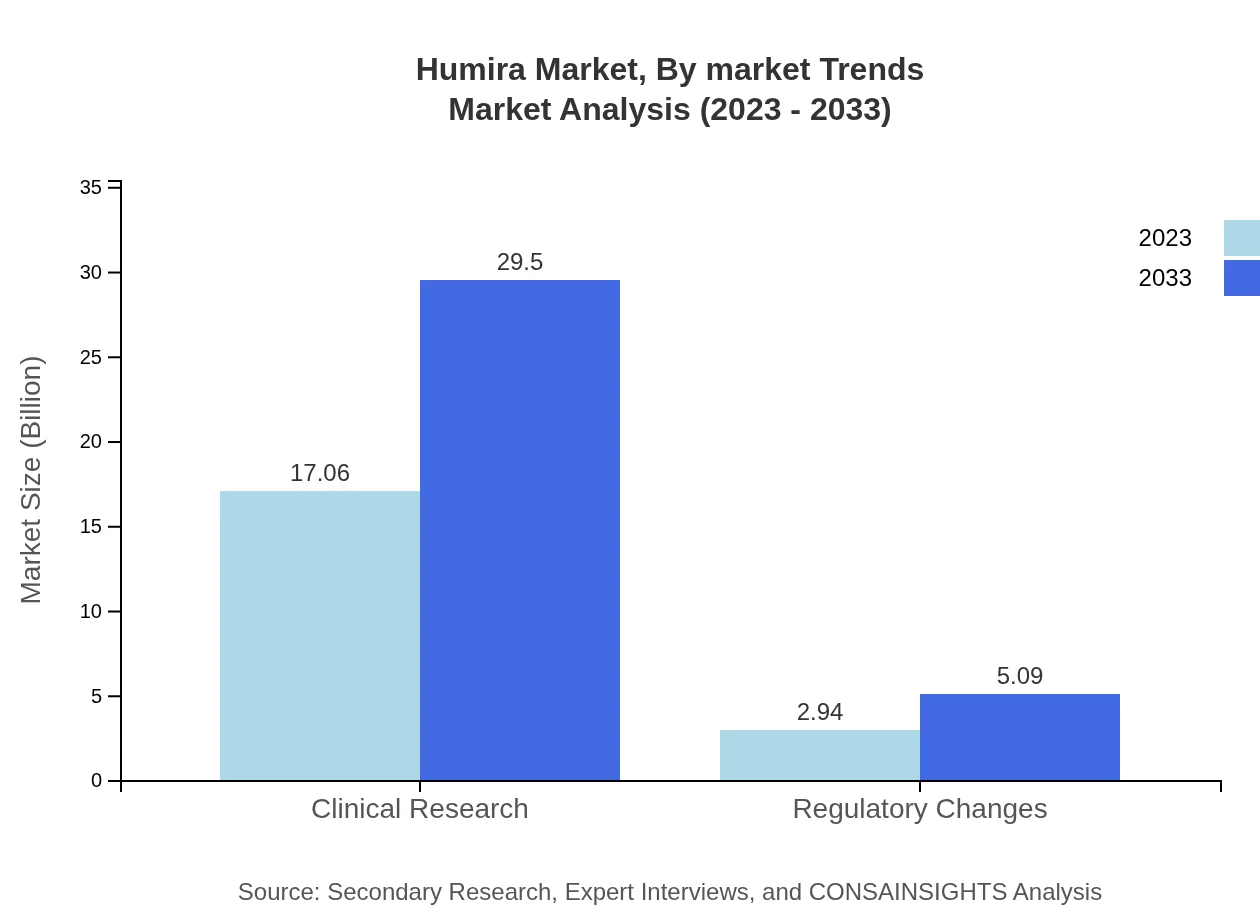
Humira Market Analysis By Market Trends
Key trends influencing the Humira market include the rise of biosimilars, developments in digital health technologies, and increased emphasis on personalized medicine. The expanding global healthcare infrastructure will also shape market dynamics, with ongoing pressure to improve affordability and access to treatments.
Humira Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Humira Industry
AbbVie:
As the original creator of Humira, AbbVie has driven its success through extensive research and marketing strategies, solidifying its position in the immunology sector.Amgen:
A significant player in the biotechnology space, Amgen focuses on developing biosimilars that challenge Humira’s market dominance, influencing pricing structures in the industry.Sandoz:
A leader in generic pharmaceuticals, Sandoz is involved in producing and distributing high-quality biosimilars, enhancing accessibility of treatments comparable to Humira.Pfizer :
With its extensive pharmaceutical portfolio, Pfizer is looking to capture market share through innovative therapies and an expanding biosimilars pipeline aimed at competing with Humira.We're grateful to work with incredible clients.









FAQs
What is the market size of Humira?
The global market size for Humira was estimated at $20 billion in 2023, with a compound annual growth rate (CAGR) of 5.5%. Projections indicate potential growth, leading to increased demand and revenue over the coming years.
What are the key market players or companies in the Humira industry?
Key players in the Humira market include leading pharmaceutical companies specializing in biologics and immunology. These firms drive innovation and maintain significant market shares, crucial for developing competitive strategies in the therapy market.
What are the primary factors driving the growth in the Humira industry?
Growth in the Humira market is primarily fueled by rising incidences of autoimmune diseases, advancing healthcare infrastructure, and increased investments in research and development. These elements contribute to higher demand for effective treatments in the therapeutic landscape.
Which region is the fastest Growing in the Humira market?
The Asia-Pacific region is anticipated to be the fastest-growing market for Humira, with its market size projected to increase from $3.96 billion in 2023 to $6.85 billion by 2033, showcasing significant regional growth and expanding healthcare access.
Does ConsInsights provide customized market report data for the Humira industry?
Yes, ConsInsights offers tailored market report data for the Humira industry, accommodating specific client needs. This customization enhances insights and assists businesses in crafting strategies tailored to their unique market objectives.
What deliverables can I expect from this Humira market research project?
From the Humira market research project, clients can expect detailed reports including market size, growth forecasts, segmentation analysis, competitive landscape insights, and regional trends, providing a comprehensive view for informed decision-making.
What are the market trends of Humira?
Current trends in the Humira market include the rise of personalized medicine, increasing generic competition, and growing focus on combination therapies. These trends drive innovation while also presenting challenges for maintaining market share.