Hunter Syndrome Treatment Market Report
Published Date: 31 January 2026 | Report Code: hunter-syndrome-treatment
Hunter Syndrome Treatment Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Hunter Syndrome Treatment market, covering insights into market size, growth forecasts, regional dynamics, and competitive landscape from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
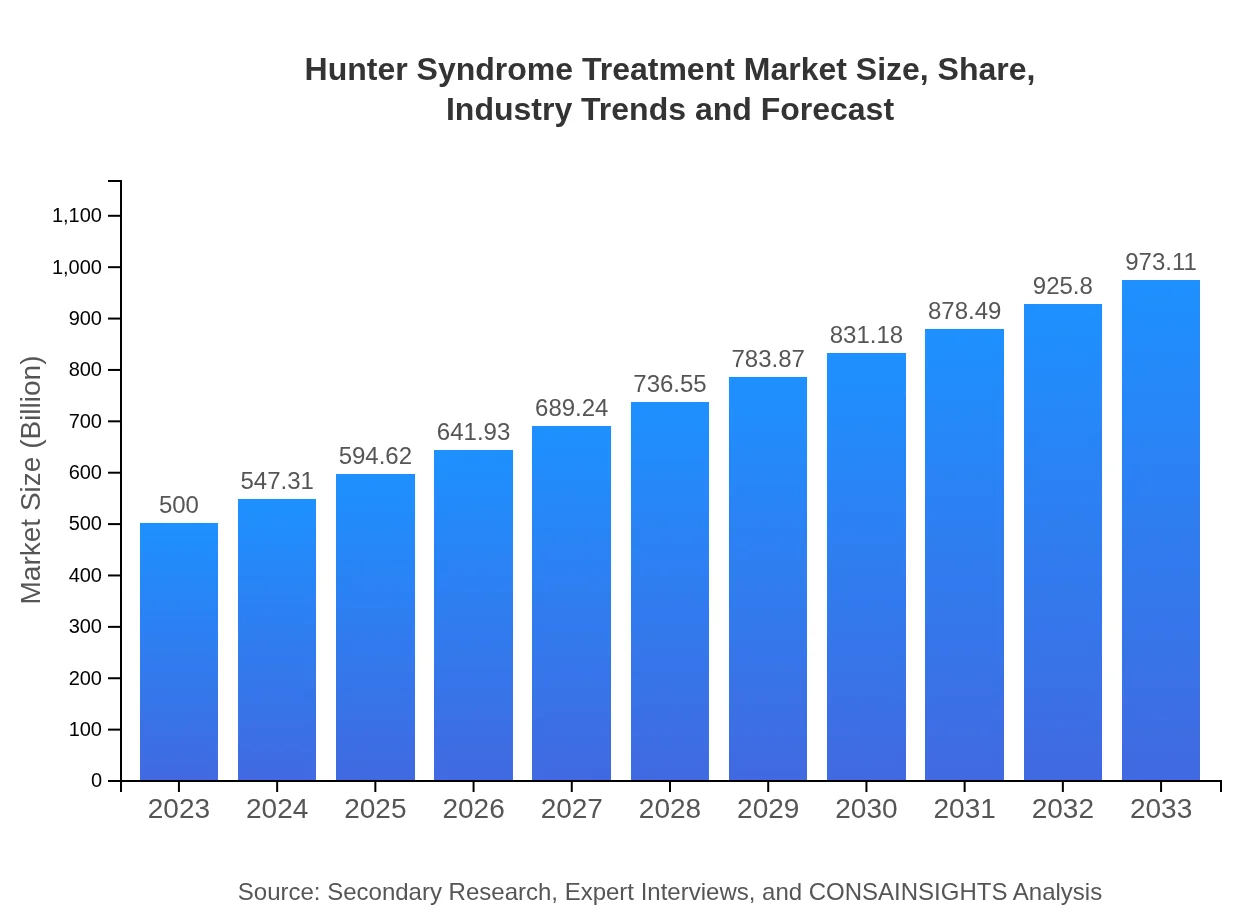
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $973.11 Million |
| Top Companies | Shire (Acquired by Takeda), Sanofi Genzyme, Ultragenyx Pharmaceutical |
| Last Modified Date | 31 January 2026 |
Hunter Syndrome Treatment Market Overview
Customize Hunter Syndrome Treatment Market Report market research report
- ✔ Get in-depth analysis of Hunter Syndrome Treatment market size, growth, and forecasts.
- ✔ Understand Hunter Syndrome Treatment's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hunter Syndrome Treatment
What is the Market Size & CAGR of Hunter Syndrome Treatment market in 2023?
Hunter Syndrome Treatment Industry Analysis
Hunter Syndrome Treatment Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hunter Syndrome Treatment Market Analysis Report by Region
Europe Hunter Syndrome Treatment Market Report:
In Europe, the market is valued at $134.95 million in 2023, projected to reach $262.64 million by 2033. The presence of advanced healthcare systems and active research institutions plays a pivotal role in driving innovative treatment strategies and increasing patient access to Hunter Syndrome therapies.Asia Pacific Hunter Syndrome Treatment Market Report:
In 2023, the Hunter Syndrome Treatment market in the Asia Pacific region is valued at $105.75 million, with projections of reaching $205.81 million by 2033. Rising healthcare expenditures and improving diagnostic capabilities are key factors contributing to this growth. Enhanced collaborations between local biotech firms and international pharmaceutical companies are also anticipated to facilitate advancements in treatment access.North America Hunter Syndrome Treatment Market Report:
The North American market is the largest, with a valuation of $169.85 million in 2023, set to expand to $330.56 million by 2033. The robust pipeline of innovative therapies and strong regulatory support for orphan drugs are primary drivers of growth in this region.South America Hunter Syndrome Treatment Market Report:
The South American market is anticipated to start at $26 million in 2023, with expectations to grow to approximately $50.60 million by 2033. Challenges regarding healthcare infrastructure and access to therapies remain, but increasing awareness and government initiatives aim to enhance the availability of treatments for Hunter Syndrome.Middle East & Africa Hunter Syndrome Treatment Market Report:
The Middle East and Africa market stands at $63.45 million in 2023 and is projected to grow to $123.49 million by 2033. Despite economic challenges, regional healthcare initiatives aimed at rare diseases are expected to enhance market potential and expand treatment accessibility.Tell us your focus area and get a customized research report.
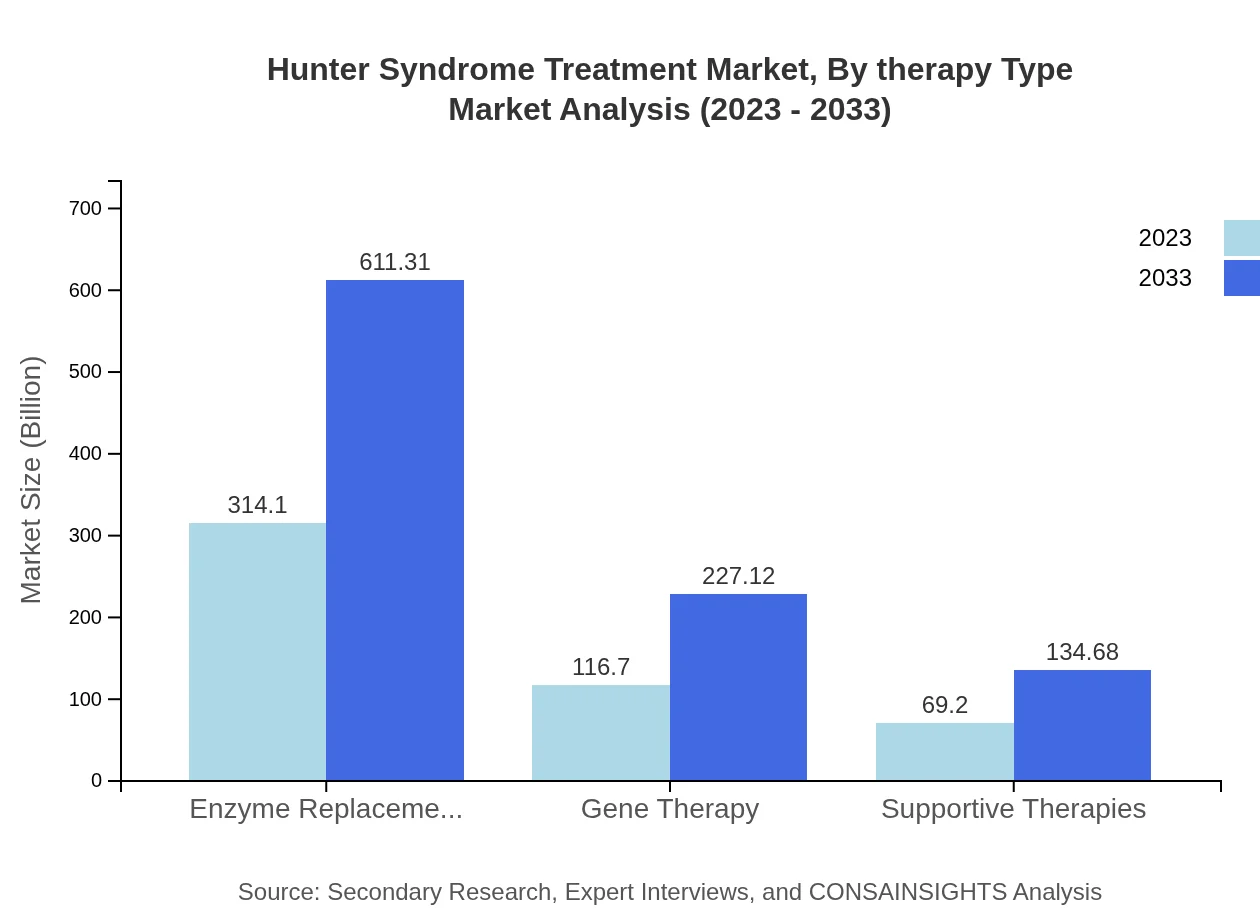
Hunter Syndrome Treatment Market Analysis By Therapy Type
The Hunter Syndrome Treatment market by therapy type is dominated by enzyme replacement therapy, valued at $314.10 million in 2023 and projected to reach $611.31 million by 2033, making up 62.82% of the market share. Gene therapy is expected to grow significantly from $116.70 million in 2023 to $227.12 million by 2033, holding a 23.34% market share. Supportive therapies also play a role, increasing from $69.20 million to $134.68 million during the same period with a 13.84% share.
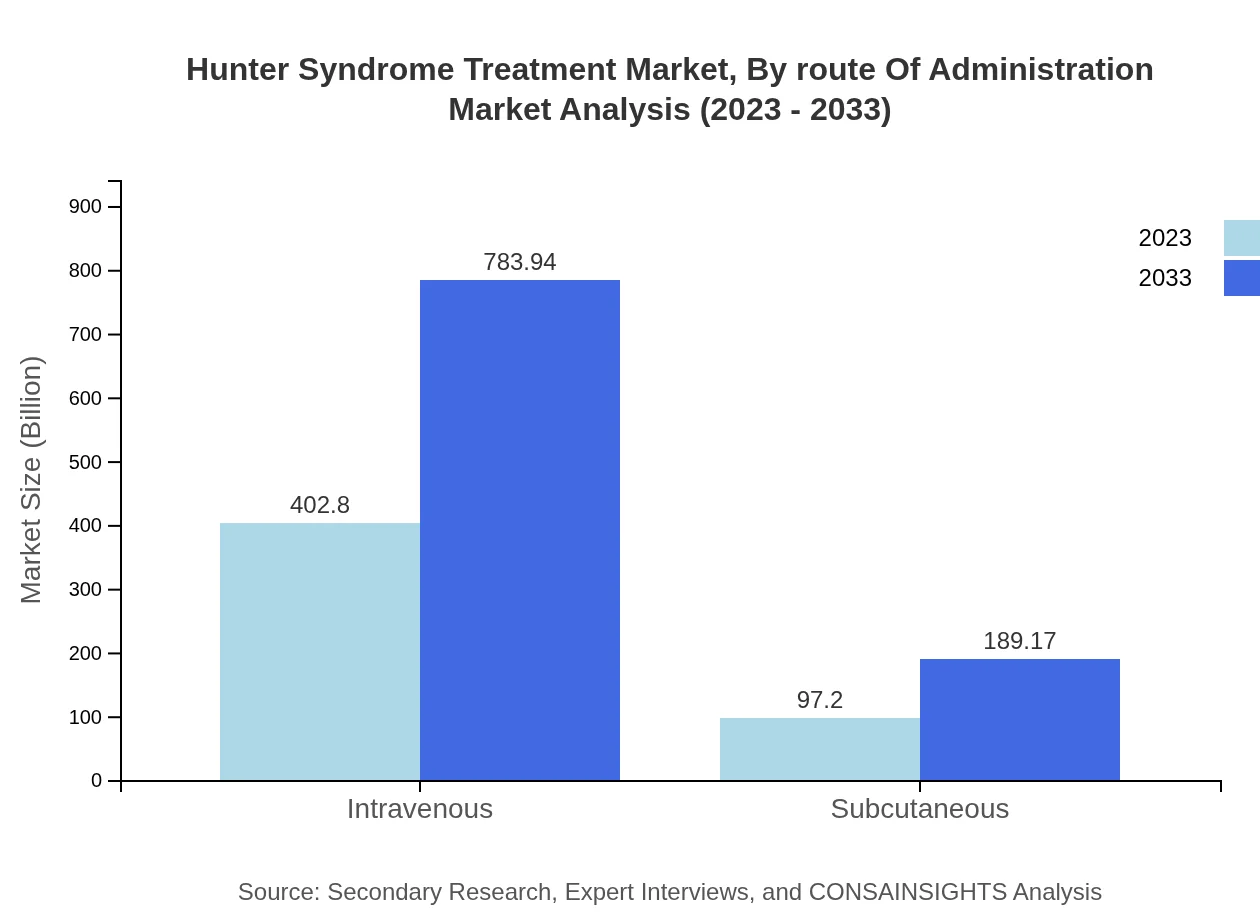
Hunter Syndrome Treatment Market Analysis By Route Of Administration
Administration methods for Hunter Syndrome treatments are crucial for patient adherence and therapy effectiveness. Intravenous administration is the most prevalent route, valued at $402.80 million in 2023 and projected to reach $783.94 million by 2033, representing an 80.56% market share. Subcutaneous administration is growing, expected to rise from $97.20 million to $189.17 million with a 19.44% share, reflecting evolving preferences in treatment administration.
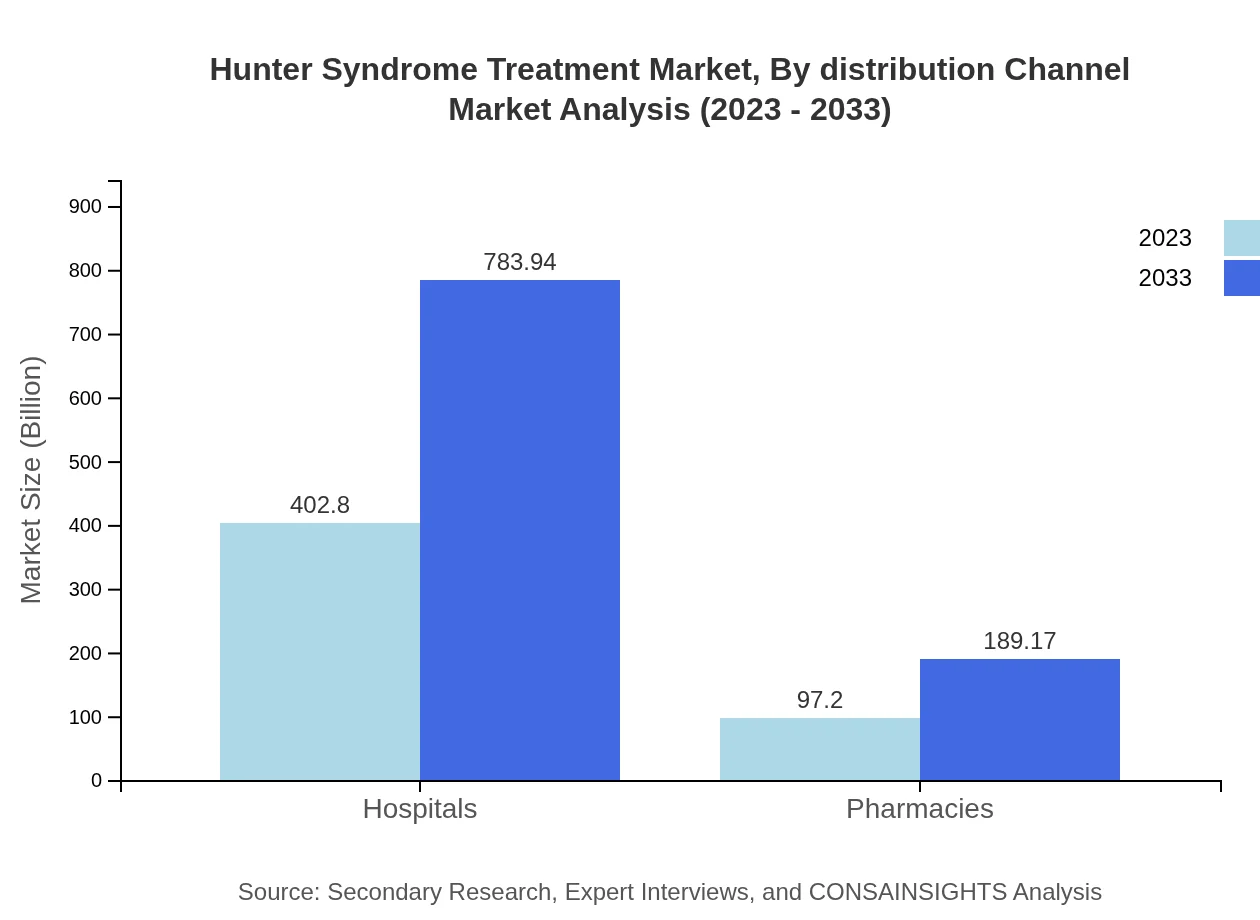
Hunter Syndrome Treatment Market Analysis By Distribution Channel
Distribution channels are vital in ensuring therapy availability. Hospitals dominate this space, starting at $402.80 million in 2023 and projected to reach $783.94 million by 2033, covering 80.56% of the market share. Pharmacies also contribute significantly, expanding from $97.20 million in 2023 to $189.17 million by 2033, accounting for 19.44% of the overall market.
Hunter Syndrome Treatment Market Analysis By Region
Global Hunter Syndrome Treatment Market, By Region Market Analysis (2023 - 2033)
Regionally, the North American and European markets lead in both size and growth. Asia Pacific shows promise with substantial growth forecasts, while Latin America and the Middle East and Africa present emerging opportunities amidst infrastructural challenges. The overall global market dynamics indicate a trend towards increased investment in therapies and heightened awareness regarding rare diseases, enhancing access and treatment quality.
Hunter Syndrome Treatment Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hunter Syndrome Treatment Industry
Shire (Acquired by Takeda):
Pioneers in enzyme replacement therapy, Shire has significantly contributed to the development of Hunter Syndrome treatments and continues to innovate for better patient outcomes.Sanofi Genzyme:
Specializes in developing therapies for rare diseases, including innovative approaches in gene therapy and enzyme replacement, enhancing treatment accessibility for patients.Ultragenyx Pharmaceutical:
Focused on genetic disease therapies, Ultragenyx is advancing potential treatment solutions tailored specifically for patients with Hunter Syndrome.We're grateful to work with incredible clients.









FAQs
What is the market size of Hunter Syndrome Treatment?
The Hunter Syndrome Treatment market is estimated to reach approximately $500 million by 2023, with a projected CAGR of 6.7%, indicating growth driven by advancements in treatment options and increasing patient awareness over the next decade.
What are the key market players or companies in the Hunter Syndrome Treatment industry?
Key players in the Hunter Syndrome Treatment market include Shire Pharmaceuticals, BioMarin Pharmaceutical, and Lucentis. They are focusing on innovative therapies and collaborations to enhance treatment availability and improve patient quality of life.
What are the primary factors driving the growth in the Hunter Syndrome Treatment industry?
Growth in the Hunter Syndrome Treatment industry is driven by increasing prevalence of Hunter Syndrome, advancements in enzyme replacement therapies, ongoing research towards gene therapy solutions, and heightened awareness and diagnosis rates among healthcare professionals.
Which region is the fastest Growing in the Hunter Syndrome Treatment?
The fastest-growing region in the Hunter Syndrome Treatment market is North America, with the market projected to grow from $169.85 million in 2023 to $330.56 million by 2033, reflecting rapid advancements in treatment options and healthcare infrastructure.
Does ConsaInsights provide customized market report data for the Hunter Syndrome Treatment industry?
Yes, ConsaInsights offers customized market report data for the Hunter Syndrome Treatment industry. Tailored reports can provide deeper insights, focusing on specific market segments, regional analyses, and trend forecasts to meet unique business needs.
What deliverables can I expect from this Hunter Syndrome Treatment market research project?
From this market research project, you can expect comprehensive market sizing data, segmentation analysis, competitive landscape insights, trend forecasts, and regional analysis, all designed to inform strategic decision-making in the Hunter Syndrome Treatment space.
What are the market trends of Hunter Syndrome Treatment?
Current trends in the Hunter Syndrome Treatment market include the rising adoption of enzyme replacement therapy, increasing investment in gene therapy research, and growing demand for holistic treatment approaches. Stakeholders emphasize innovative delivery methods to improve patient outcomes.