Hyperphosphatemia Drugs Market Report
Published Date: 31 January 2026 | Report Code: hyperphosphatemia-drugs
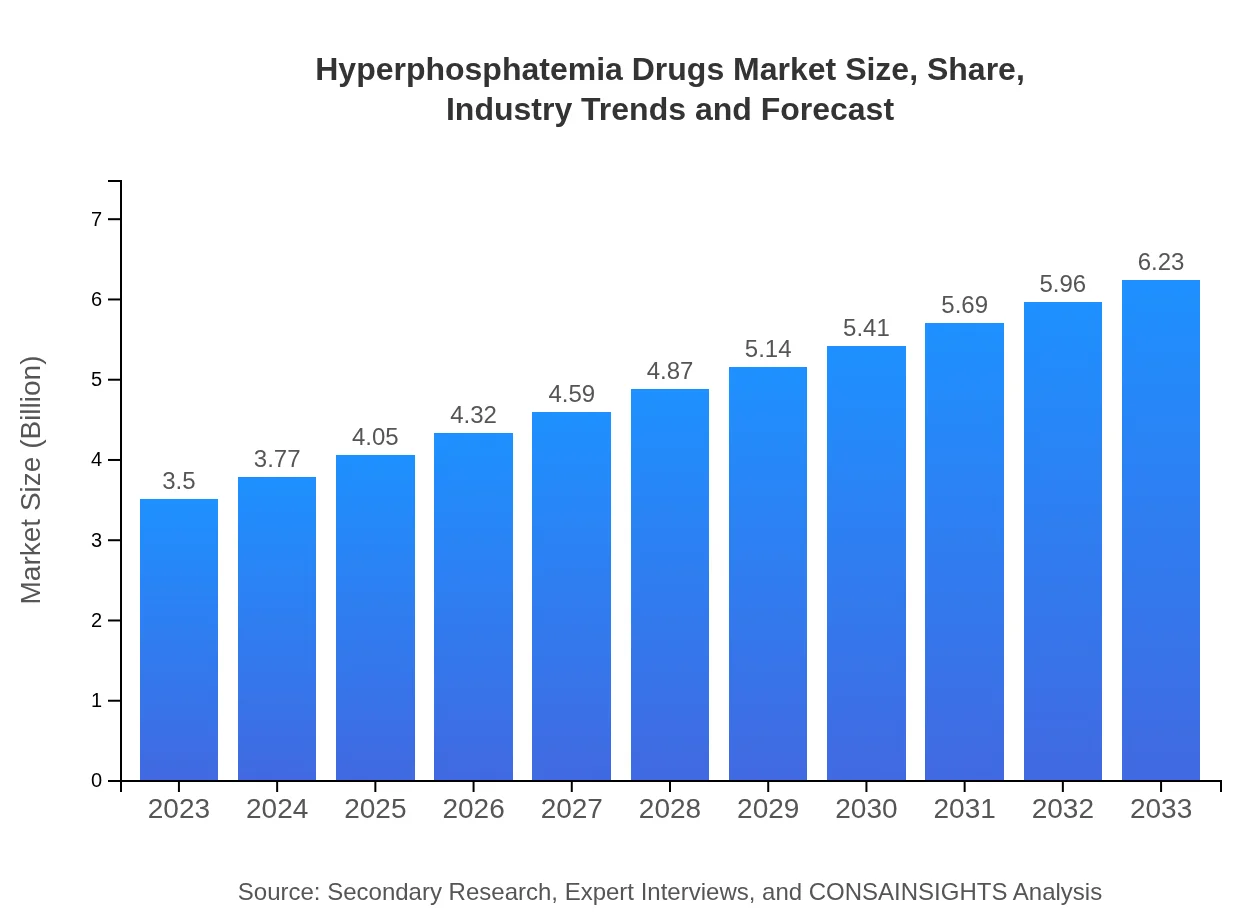
Hyperphosphatemia Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the hyperphosphatemia drugs market including market size, trends, forecasts from 2023 to 2033, and insights into regional performance and industry dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $6.23 Billion |
| Top Companies | Amgen Inc., Fresenius Medical Care, Sanofi |
| Last Modified Date | 31 January 2026 |
Hyperphosphatemia Drugs Market Overview
Customize Hyperphosphatemia Drugs Market Report market research report
- ✔ Get in-depth analysis of Hyperphosphatemia Drugs market size, growth, and forecasts.
- ✔ Understand Hyperphosphatemia Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Hyperphosphatemia Drugs
What is the Market Size & CAGR of Hyperphosphatemia Drugs market in 2023?
Hyperphosphatemia Drugs Industry Analysis
Hyperphosphatemia Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Hyperphosphatemia Drugs Market Analysis Report by Region
Europe Hyperphosphatemia Drugs Market Report:
The European market for hyperphosphatemia drugs is forecasted to increase from $1.05 billion in 2023 to $1.86 billion in 2033. Factors such as rising healthcare costs and growing aging population contribute significantly to the rising demand for effective treatments.Asia Pacific Hyperphosphatemia Drugs Market Report:
The Asia Pacific region is expected to witness considerable growth, with a market size projected to reach $1.29 billion by 2033, up from $0.72 billion in 2023. Factors contributing to this growth include a high prevalence of kidney disease and increasing health expenditure.North America Hyperphosphatemia Drugs Market Report:
North America holds a substantial share of the market, with an expected growth from $1.16 billion in 2023 to $2.06 billion by 2033. The strong presence of leading pharmaceutical companies and advanced healthcare systems drive consistent market development.South America Hyperphosphatemia Drugs Market Report:
In South America, the market is projected to grow from $0.17 billion in 2023 to $0.31 billion by 2033. The increasing focus on improving healthcare infrastructure and access to medication is essential for addressing hyperphosphatemia in this region.Middle East & Africa Hyperphosphatemia Drugs Market Report:
The Middle East and Africa market is anticipated to grow from $0.41 billion in 2023 to $0.72 billion by 2033. Improving healthcare access and government initiatives in health care funding are key drivers in this region.Tell us your focus area and get a customized research report.
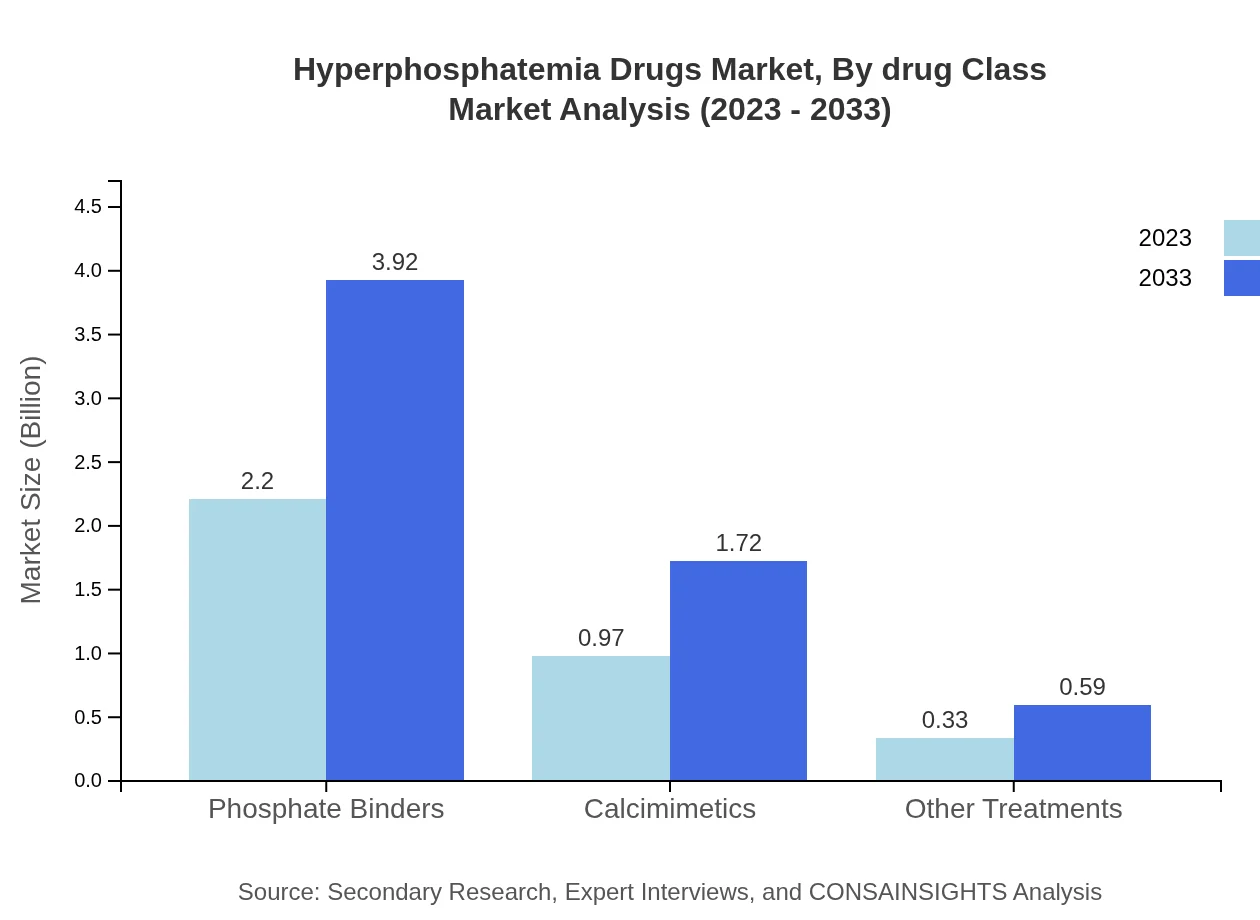
Hyperphosphatemia Drugs Market Analysis By Drug Class
Phosphate binders currently dominate the market, contributing $2.20 billion in 2023 and projected to grow to $3.92 billion by 2033, representing a market share of 62.81%. Calcimimetics, while smaller with $0.97 billion in 2023, are expected to increase to $1.72 billion by 2033, maintaining a 27.67% market share. Other treatments contribute $0.33 billion in 2023, growing to $0.59 billion by 2033 with a 9.52% share.
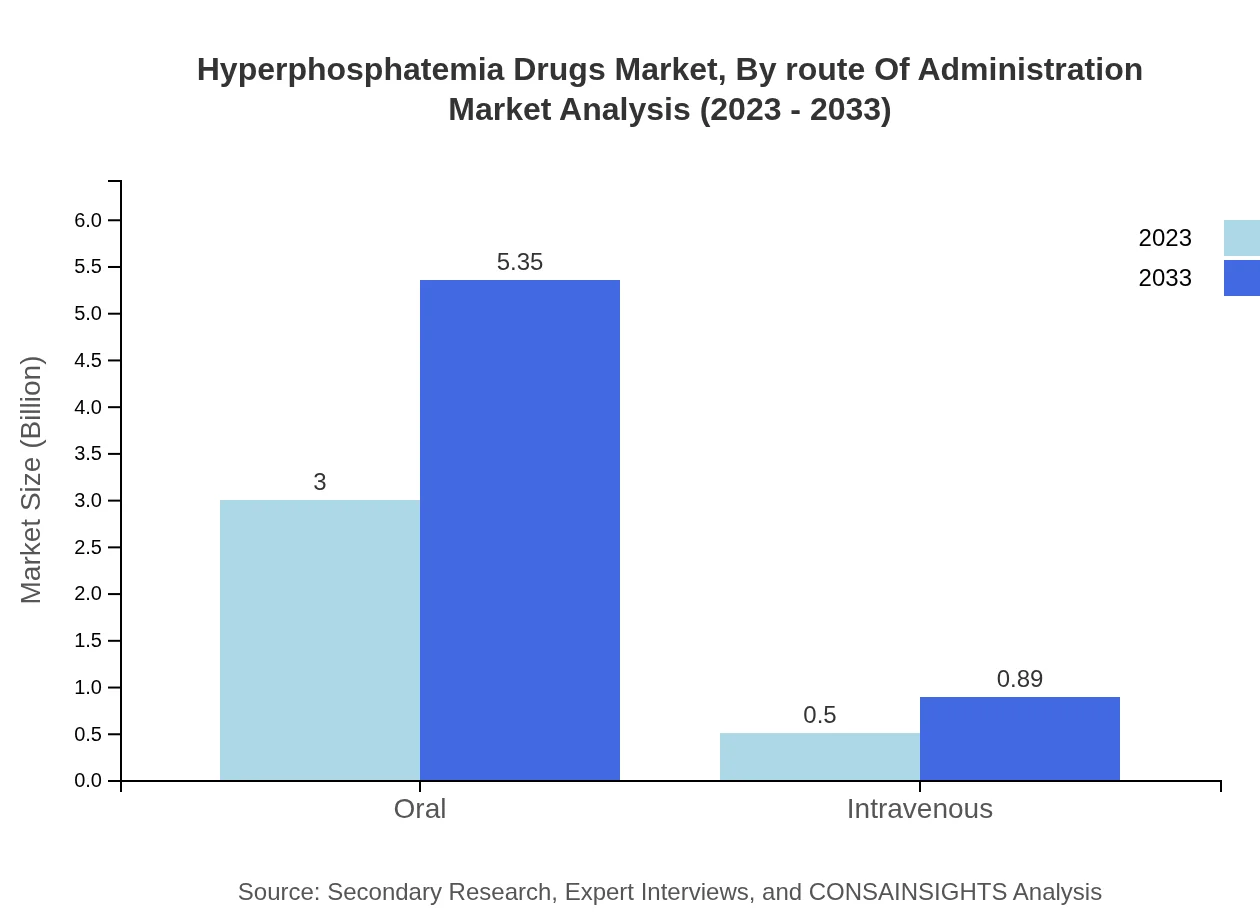
Hyperphosphatemia Drugs Market Analysis By Route Of Administration
Oral medications account for a significant majority of market share at 85.8%, with a market size expected to grow from $3.00 billion in 2023 to $5.35 billion by 2033. Intravenous administration, while less common, is also experiencing growth from $0.50 billion in 2023 to $0.89 billion, taking a 14.2% share.
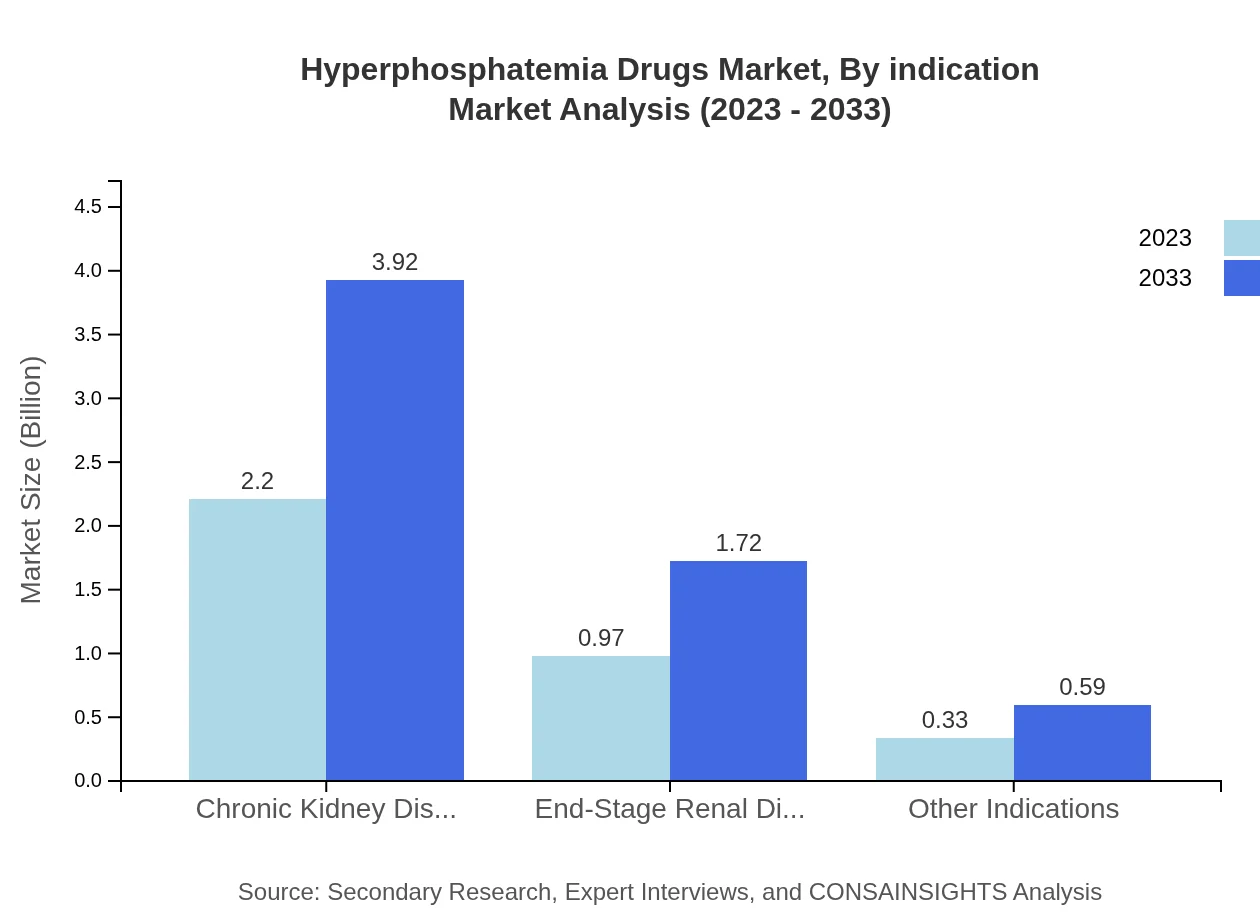
Hyperphosphatemia Drugs Market Analysis By Indication
Chronic kidney disease encompasses the largest share, with a market size of $2.20 billion in 2023 and a projected growth to $3.92 billion by 2033. End-stage renal disease treatments are also growing, from $0.97 billion to $1.72 billion. Treatments for other indications contribute smaller but significant shares of the market.
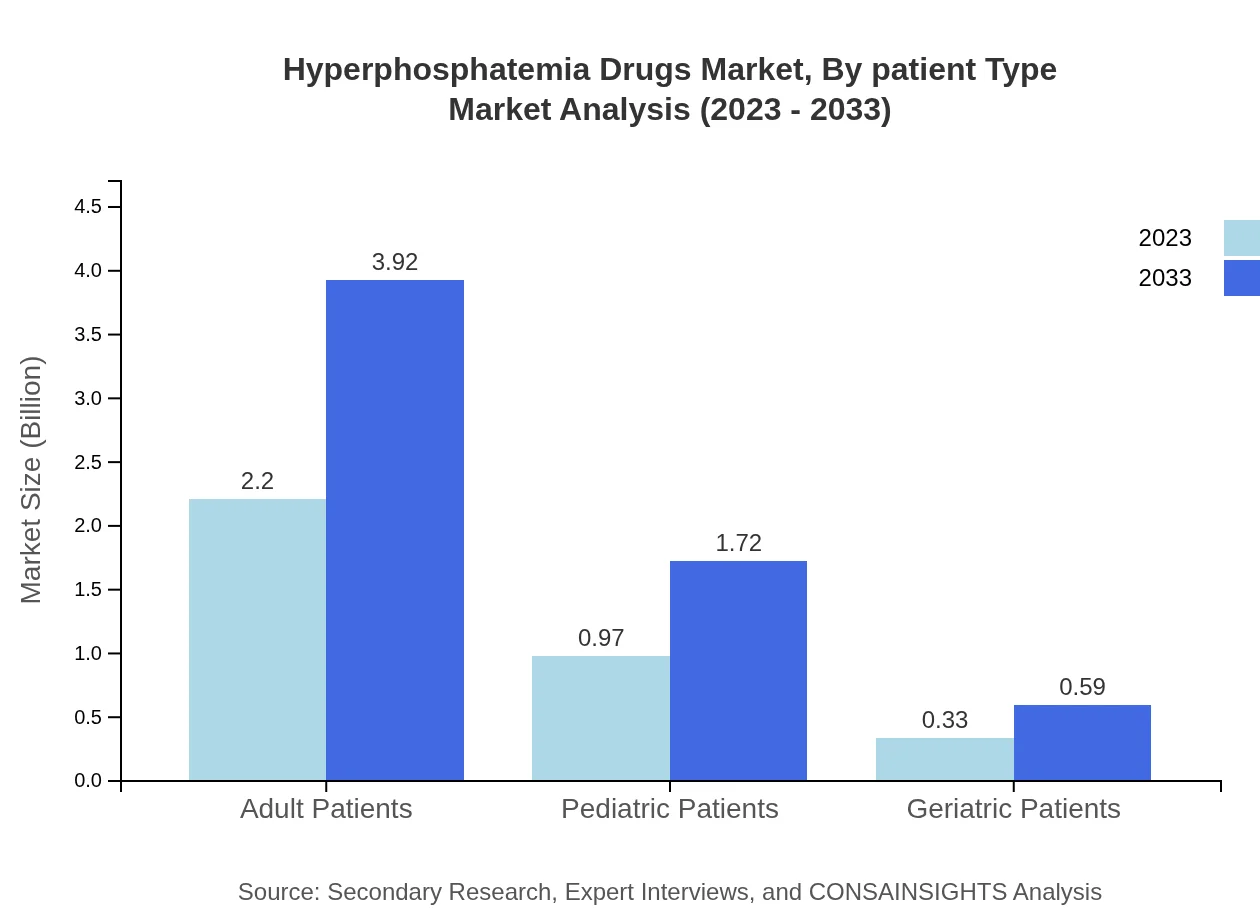
Hyperphosphatemia Drugs Market Analysis By Patient Type
Adult patients dominate the market with a size of $2.20 billion in 2023, growing to $3.92 billion by 2033, while pediatric and geriatric patients represent smaller segments with $0.97 billion and $0.33 billion in 2023, respectively, projected to grow to $1.72 billion and $0.59 billion by 2033.
Hyperphosphatemia Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Hyperphosphatemia Drugs Industry
Amgen Inc.:
Amgen Inc. specializes in biotechnology and has a leading portfolio in the development of calcimimetics, contributing significantly to the treatment options available for hyperphosphatemia.Fresenius Medical Care:
Fresenius focuses on chronic kidney disease management solutions and has developed effective phosphate binders that are widely used among ESRD patients.Sanofi:
Sanofi is involved in the development of several therapeutic drugs for managing phosphate levels, driving innovation in the hyperphosphatemia space.We're grateful to work with incredible clients.









FAQs
What is the market size of hyperphosphatemia Drugs?
The global hyperphosphatemia drugs market was valued at approximately $3.5 billion in 2023, with an expected CAGR of 5.8% through 2033, suggesting robust growth driven by increased incidences of related health conditions.
What are the key market players or companies in this hyperphosphatemia Drugs industry?
Key players in the hyperphosphatemia drugs market include major pharmaceutical companies that produce phosphate binders and calcimimetics. Their strategic focus on research and innovation enhances their competitive positioning within the industry.
What are the primary factors driving the growth in the hyperphosphatemia Drugs industry?
Growth in the hyperphosphatemia drugs sector is primarily driven by increasing prevalence of chronic kidney disease, technological advancements in drug formulation, and rising awareness of effective treatment options among healthcare professionals and patients.
Which region is the fastest Growing in the hyperphosphatemia Drugs?
The North American region is projected to be the fastest-growing market for hyperphosphatemia drugs, expanding from $1.16 billion in 2023 to $2.06 billion by 2033, fueled by increasing patient populations and access to healthcare services.
Does ConsaInsights provide customized market report data for the hyperphosphatemia Drugs industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs within the hyperphosphatemia drugs sector, providing actionable insights and detailed data suited for targeted market strategies.
What deliverables can I expect from this hyperphosphatemia Drugs market research project?
Expect comprehensive reports detailing market size, growth forecasts, competitive analysis, regional insights, and segment-specific data, presenting a well-rounded view of the hyperphosphatemia drugs market and its dynamics.
What are the market trends of hyperphosphatemia Drugs?
Current trends in the hyperphosphatemia drugs market highlight an increasing shift towards oral drug formulations, personalized medicine approaches, and the growing importance of patient compliance in treatment plans.