Idiopathic Pulmonary Fibrosis Market Report
Published Date: 31 January 2026 | Report Code: idiopathic-pulmonary-fibrosis
Idiopathic Pulmonary Fibrosis Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Idiopathic Pulmonary Fibrosis (IPF) market from 2023 to 2033, covering market size, growth trends, industry dynamics, and insights by region and product type.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
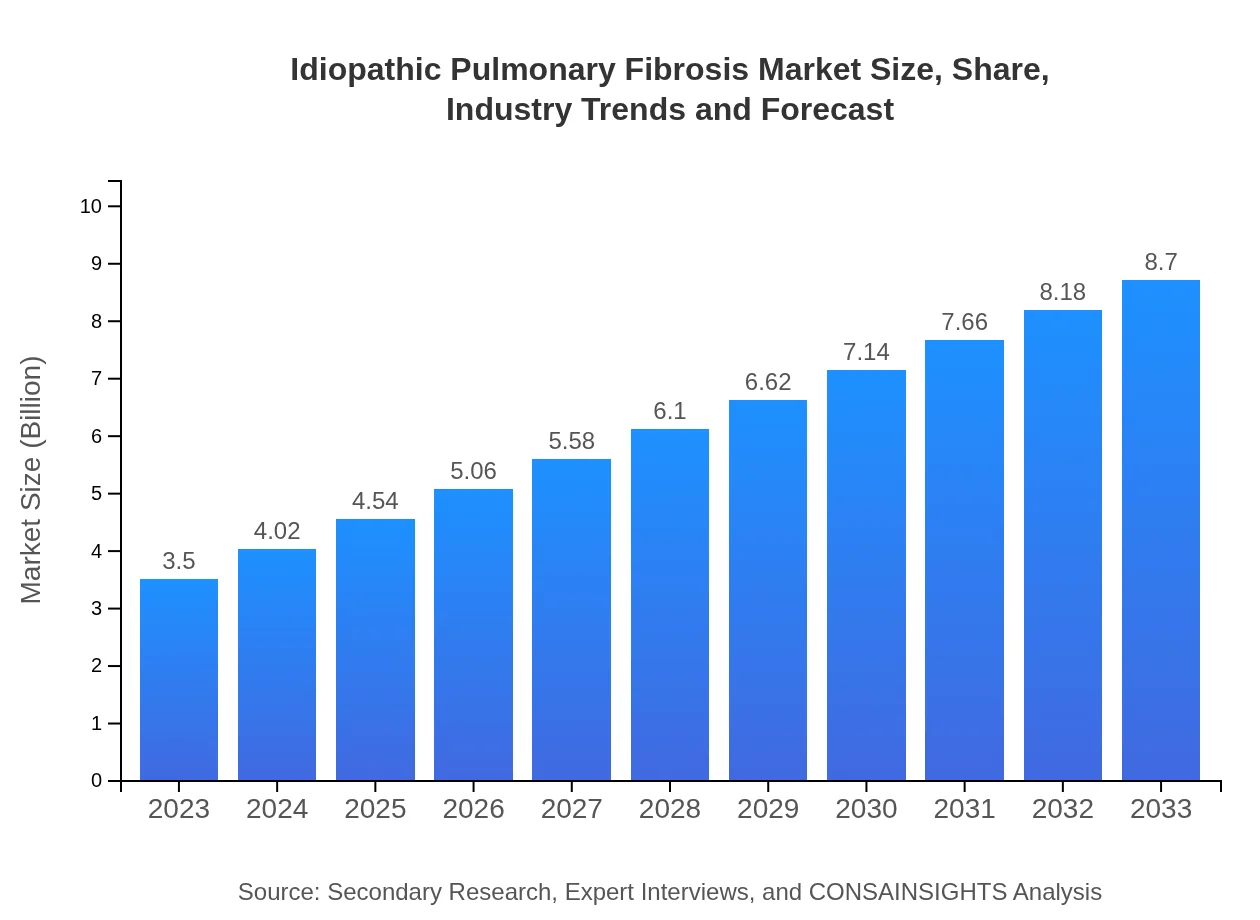
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $8.70 Billion |
| Top Companies | Boehringer Ingelheim, Roche, Galapagos, Bristol-Myers Squibb, Pfizer |
| Last Modified Date | 31 January 2026 |
Idiopathic Pulmonary Fibrosis Market Overview
Customize Idiopathic Pulmonary Fibrosis Market Report market research report
- ✔ Get in-depth analysis of Idiopathic Pulmonary Fibrosis market size, growth, and forecasts.
- ✔ Understand Idiopathic Pulmonary Fibrosis's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Idiopathic Pulmonary Fibrosis
What is the Market Size & CAGR of Idiopathic Pulmonary Fibrosis market in 2023?
Idiopathic Pulmonary Fibrosis Industry Analysis
Idiopathic Pulmonary Fibrosis Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Idiopathic Pulmonary Fibrosis Market Analysis Report by Region
Europe Idiopathic Pulmonary Fibrosis Market Report:
The European IPF market is expected to rise from $1.01 billion in 2023 to $2.51 billion by 2033. The region demonstrates strong growth due to rising awareness and supportive regulatory frameworks for drug development. The prevalence of IPF is also increasing, further driving the demand for effective treatment modalities.Asia Pacific Idiopathic Pulmonary Fibrosis Market Report:
In the Asia Pacific region, the IPF market is expected to grow from $0.69 billion in 2023 to $1.70 billion by 2033. Increasing healthcare facilities, rising awareness of lung diseases, and growing disposable incomes are contributing to this growth. The pharmaceutical market is witnessing the launch of innovative drug therapies, significantly impacting IPF treatment landscapes.North America Idiopathic Pulmonary Fibrosis Market Report:
North America holds a significant share of the IPF market, projected to grow from $1.21 billion in 2023 to $2.99 billion by 2033. The high incidence of IPF, combined with advanced healthcare infrastructure and significant investment in research, aids in market growth. Additionally, increased availability of novel treatment options aligns with the expanding patient population.South America Idiopathic Pulmonary Fibrosis Market Report:
The South American IPF market is expected to expand from $0.20 billion in 2023 to $0.49 billion by 2033. The market growth is facilitated by improving healthcare access and advancements in diagnostic systems, despite certain challenges like economic disparities affecting overall healthcare spending.Middle East & Africa Idiopathic Pulmonary Fibrosis Market Report:
The IPF market in the Middle East and Africa is projected to increase from $0.40 billion in 2023 to $0.99 billion by 2033. The market is benefitting from a growth in healthcare reforms, improvements in lung disease diagnostic capabilities, and an uptick in medical research investments in the region.Tell us your focus area and get a customized research report.
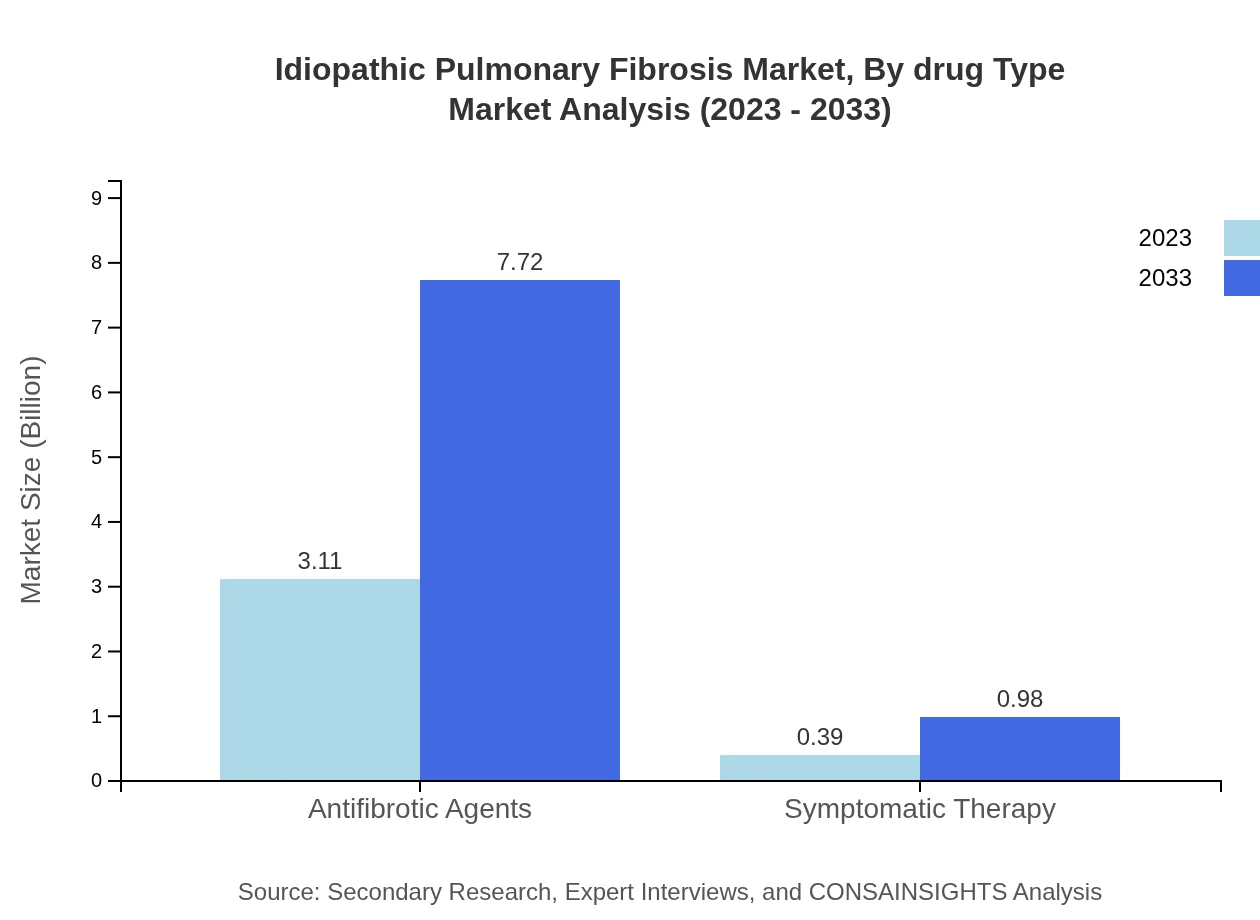
Idiopathic Pulmonary Fibrosis Market Analysis By Drug Type
The IPF drug market primarily comprises antifibrotic agents, accounting for $3.11 billion in 2023, expected to rise to $7.72 billion by 2033. Antifibrotic drugs hold an 88.76% market share, dominating treatment paradigms. Symptomatic therapies, while growing, represent a smaller market share of 11.24%, projected to grow from $0.39 billion in 2023 to $0.98 billion by 2033.
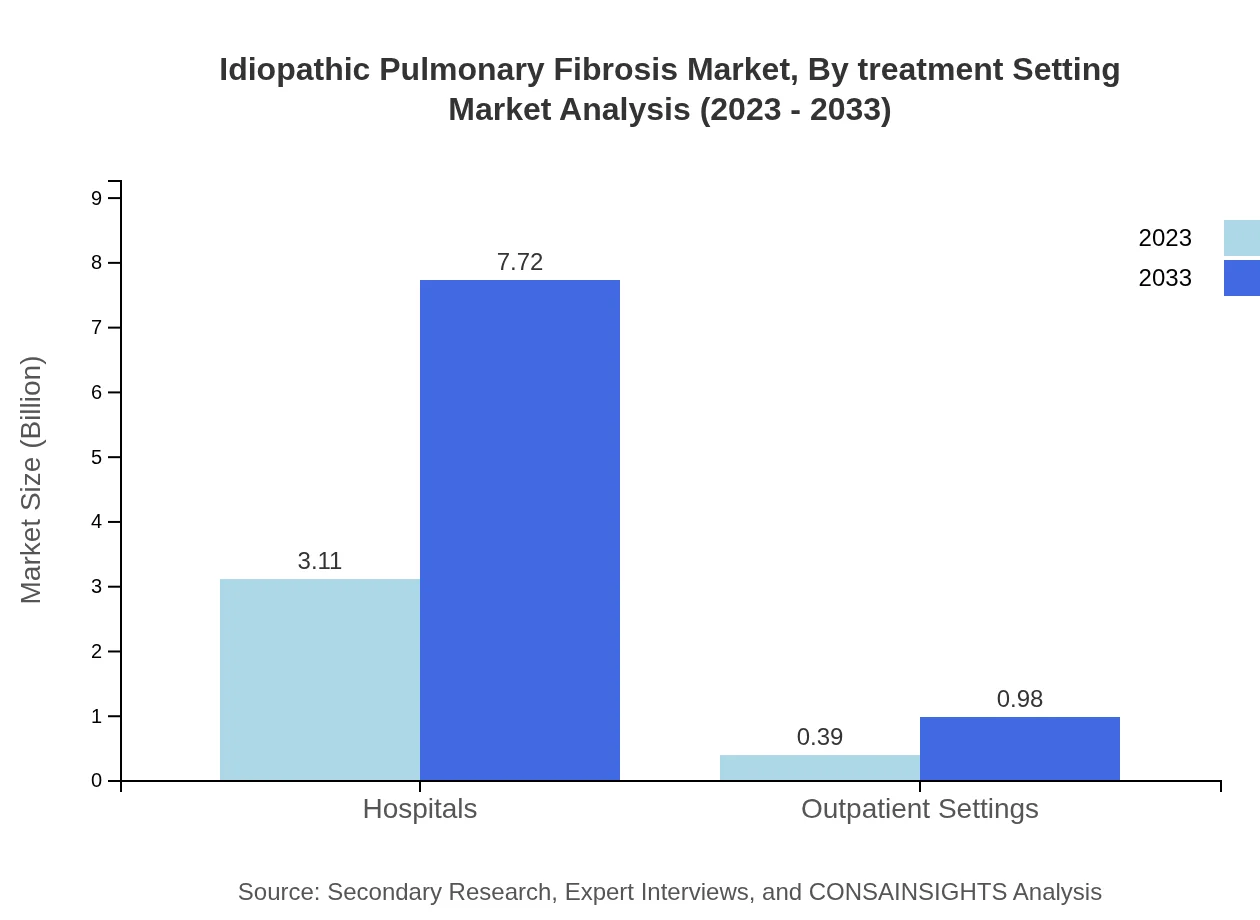
Idiopathic Pulmonary Fibrosis Market Analysis By Treatment Setting
Treatment settings for IPF include hospitals, clinics, and home care settings. Hospitals dominate this segment with a market size of $3.11 billion in 2023, growing to $7.72 billion by 2033, reflecting the need for comprehensive treatment and emergency care. Clinics are projected to increase from $0.76 billion in 2023 to $1.90 billion by 2033. Home care settings, albeit smaller, are also climbing from $0.36 billion to $0.89 billion due to trends toward patient-centered care.
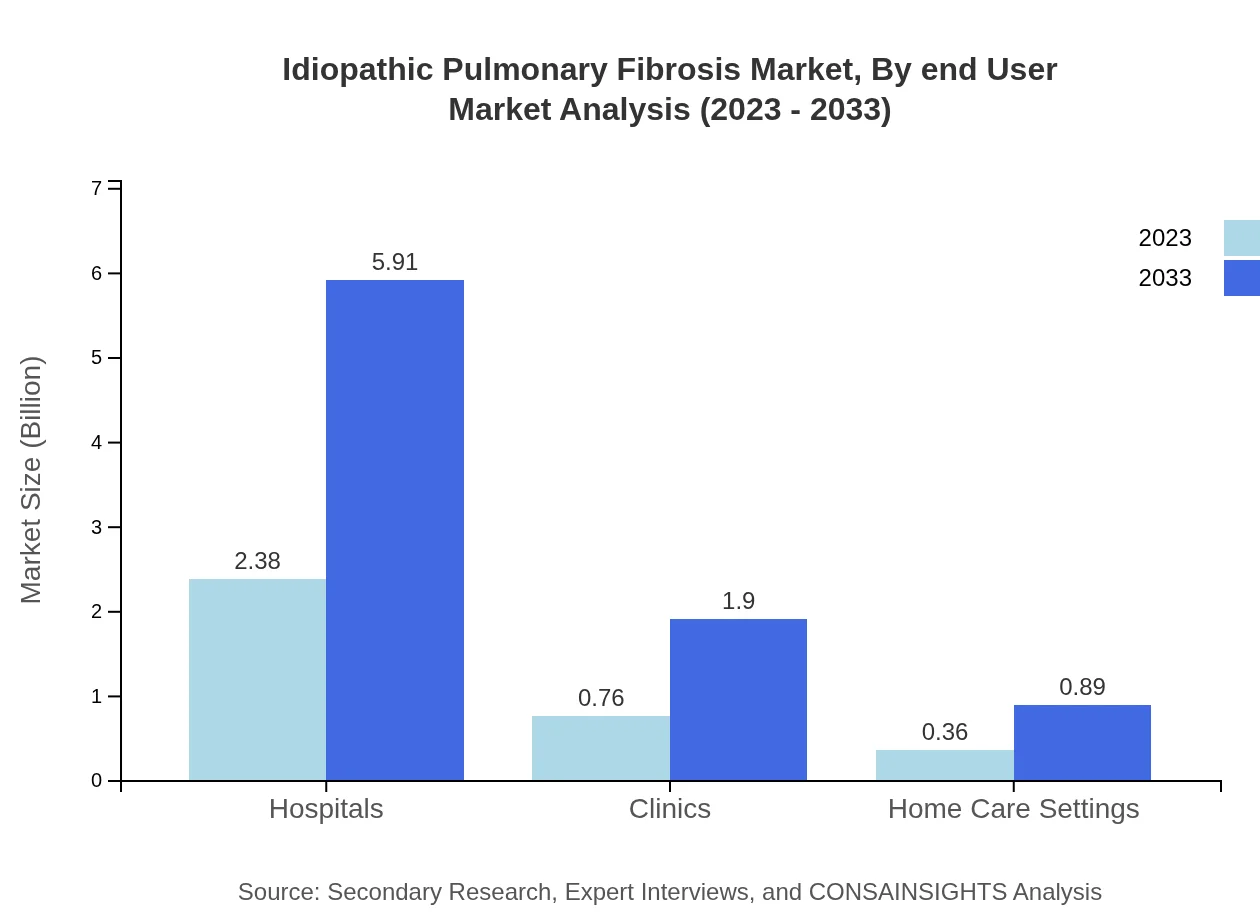
Idiopathic Pulmonary Fibrosis Market Analysis By End User
In terms of end-users, hospitals continue to lead the market with significant contributions to treatment strategies. The growing prevalence of IPF and complex management needs increases hospital visits. Outpatient settings are also emerging as important venues for managing IPF, expected to grow from $0.39 billion in 2023 to $0.98 billion by 2033.
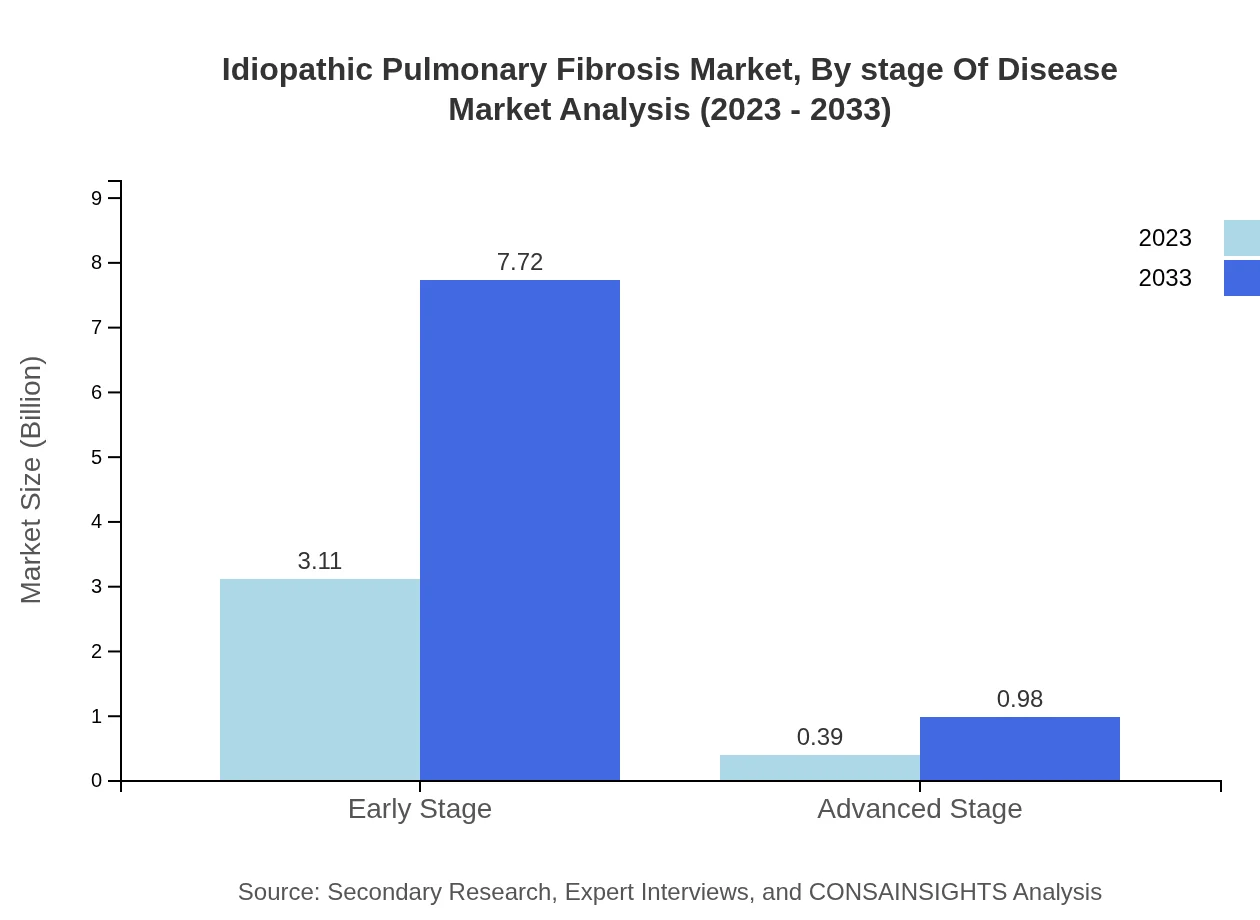
Idiopathic Pulmonary Fibrosis Market Analysis By Stage Of Disease
IPF management is critical at both early and advanced stages of disease. Early-stage treatment accounts for 88.76% of the market share, expected to increase from $3.11 billion to $7.72 billion by 2033. Advanced-stage management strategies occupy the remaining share, growing from $0.39 billion to $0.98 billion, highlighting the importance of timely intervention.
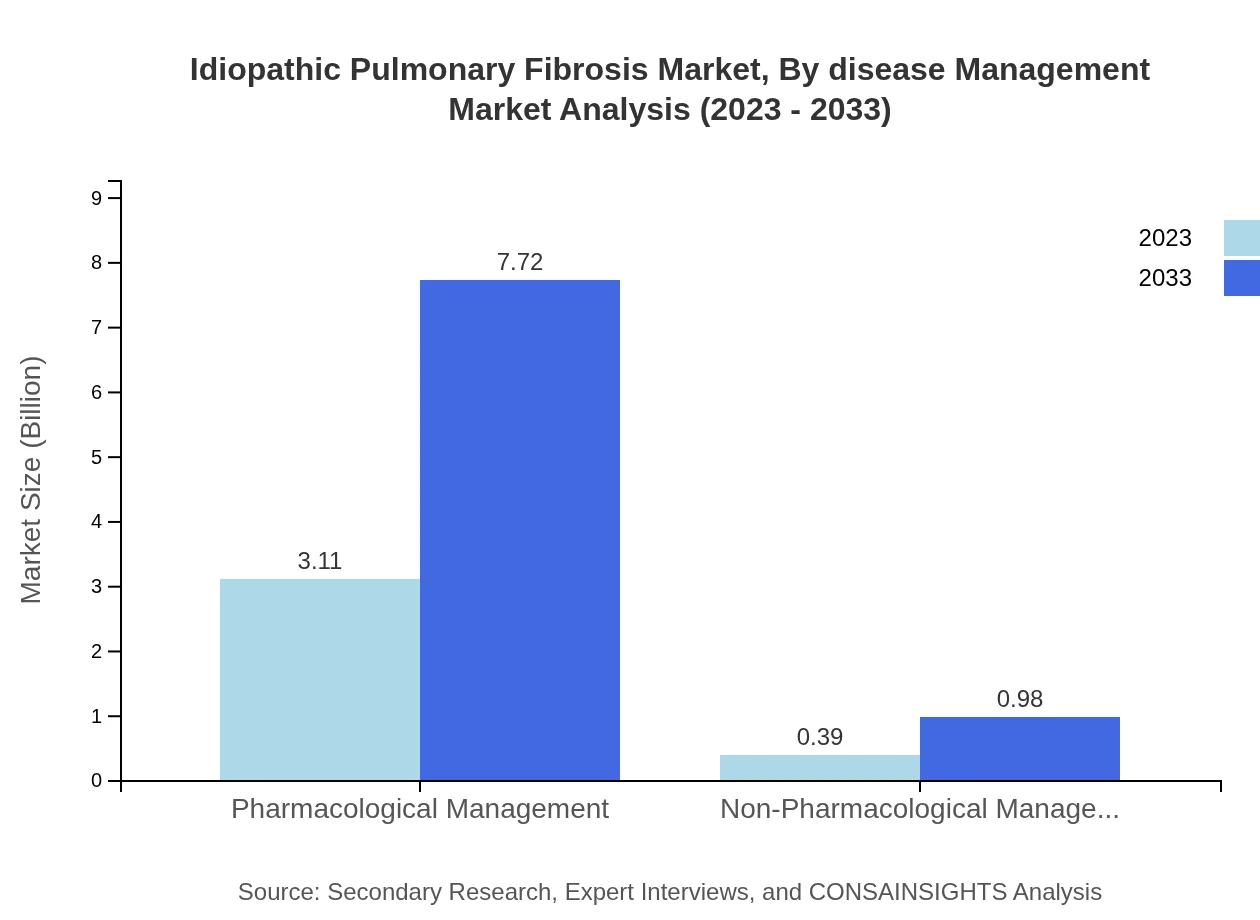
Idiopathic Pulmonary Fibrosis Market Analysis By Disease Management
Disease management for IPF combines pharmacological and non-pharmacological approaches. The pharmacological management segment commands 88.76% of the market, projected to grow significantly. Non-pharmacological interventions, while smaller (11.24%), are gaining traction for improving patient quality of life and supporting disease progression.
Idiopathic Pulmonary Fibrosis Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Idiopathic Pulmonary Fibrosis Industry
Boehringer Ingelheim:
A global pharmaceutical company leading in IPF treatment with its drug, Ofev (Nintedanib), a key antifibrotic therapy.Roche:
Developers of Esbriet (Pirfenidone), a breakthrough medication that significantly impacts the treatment of IPF.Galapagos:
Focused on developing innovative treatments for IPF, including investigational drugs aimed at different pathways in fibrosis.Bristol-Myers Squibb:
Engaged in research for the development of IPF therapeutics, emphasizing on improving patient outcomes.Pfizer :
Involved in multiple clinical trials aimed at optimizing treatment options for patients with IPF.We're grateful to work with incredible clients.









FAQs
What is the market size of idiopathic Pulmonary Fibrosis?
The idiopathic pulmonary fibrosis market size is projected to reach approximately $3.5 billion by 2033, growing at a CAGR of 9.2%. This growth indicates a rising demand for effective treatments and therapies in this crucial healthcare sector.
What are the key market players or companies in this idiopathic Pulmonary Fibrosis industry?
Key players include major pharmaceutical companies specializing in pulmonary disease management, particularly focusing on antifibrotic agents. Their ongoing R&D efforts are pivotal in addressing market needs and expanding treatment options.
What are the primary factors driving the growth in the idiopathic Pulmonary Fibrosis industry?
Growth factors include the increasing prevalence of IPF, advancements in medical therapies, and a heightened focus on personalized medicine. Awareness campaigns also play a significant role in facilitating early diagnosis and treatment.
Which region is the fastest Growing in the idiopathic Pulmonary Fibrosis market?
The fastest-growing region is North America, with projected market growth from $1.21 billion in 2023 to $2.99 billion by 2033. Europe also shows substantial growth potential in the same period, enhancing overall market dynamics.
Does ConsaInsights provide customized market report data for the idiopathic Pulmonary Fibrosis industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs within the idiopathic pulmonary fibrosis sector, enabling stakeholders to gain precise insights that align with their business strategies and objectives.
What deliverables can I expect from this idiopathic Pulmonary Fibrosis market research project?
The deliverables typically include comprehensive market analysis, detailed forecasts, competitive landscape assessments, and segment-specific insights tailored to your requirements, aiding in strategic decision-making.
What are the market trends of idiopathic Pulmonary Fibrosis?
Current trends indicate a shift towards targeted therapies, increased investment in R&D for novel treatment modalities, and rising emphasis on patient-centric care strategies to improve outcomes for individuals affected by IPF.