Immune Checkpoint Inhibitors Market Report
Published Date: 31 January 2026 | Report Code: immune-checkpoint-inhibitors
Immune Checkpoint Inhibitors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Immune Checkpoint Inhibitors market, covering size, growth trends, segmentation, and key market players. Insights are projected from 2023 to 2033, offering valuable information for stakeholders in the pharmaceutical industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
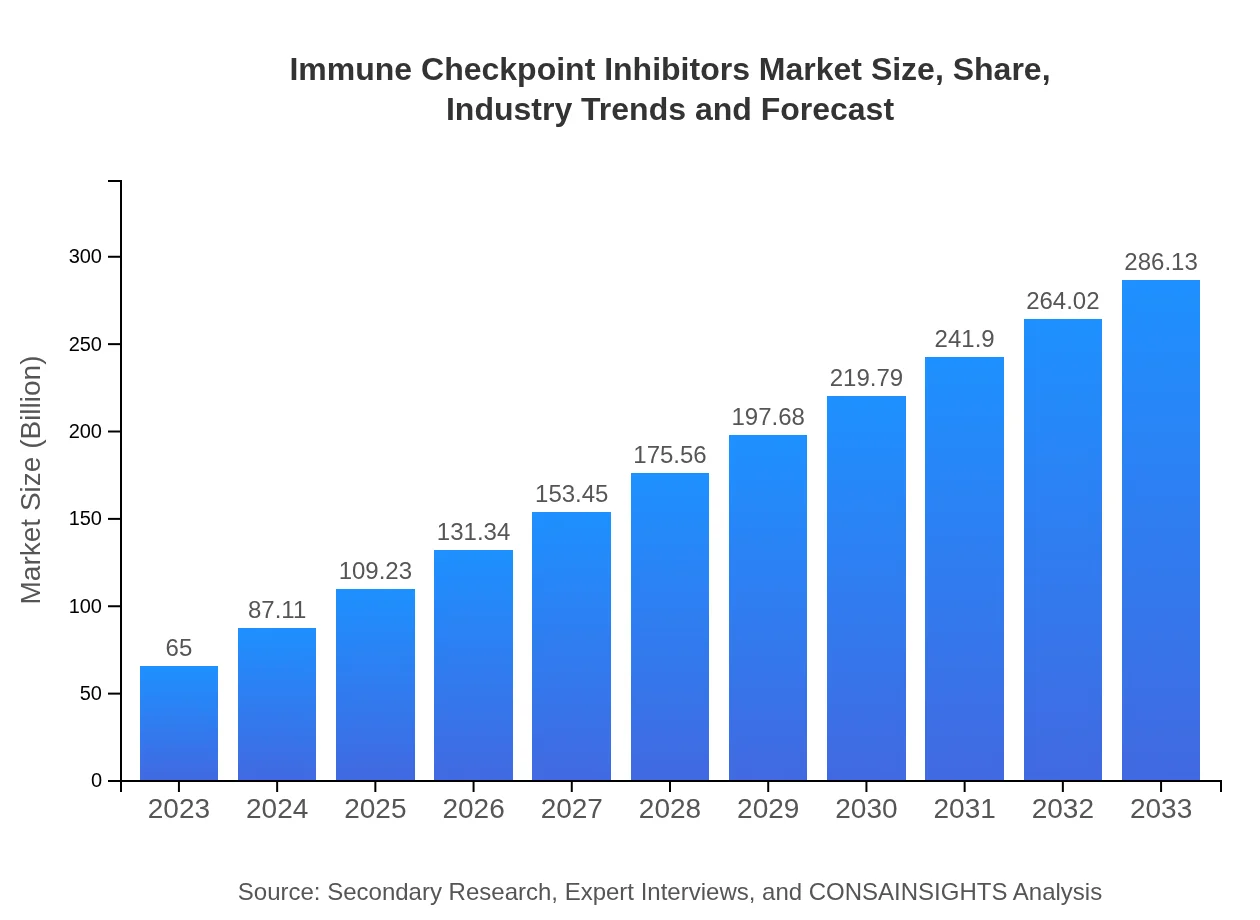
| 2023 Market Size | $65.00 Billion |
| CAGR (2023-2033) | 15.2% |
| 2033 Market Size | $286.13 Billion |
| Top Companies | Bristol-Myers Squibb, Merck & Co., Roche, AstraZeneca, Pfizer |
| Last Modified Date | 31 January 2026 |
Immune Checkpoint Inhibitors Market Overview
Customize Immune Checkpoint Inhibitors Market Report market research report
- ✔ Get in-depth analysis of Immune Checkpoint Inhibitors market size, growth, and forecasts.
- ✔ Understand Immune Checkpoint Inhibitors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Immune Checkpoint Inhibitors
What is the Market Size & CAGR of Immune Checkpoint Inhibitors market in 2023 and 2033?
Immune Checkpoint Inhibitors Industry Analysis
Immune Checkpoint Inhibitors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Immune Checkpoint Inhibitors Market Analysis Report by Region
Europe Immune Checkpoint Inhibitors Market Report:
The European market is projected to grow from USD 21.06 billion in 2023 to USD 92.71 billion by 2033. The region is proactive in oncology drug approvals and has robust public health policies promoting cancer treatment access, which fosters market growth.Asia Pacific Immune Checkpoint Inhibitors Market Report:
The Asia Pacific region's Immune Checkpoint Inhibitors market is projected to grow, reaching USD 51.56 billion by 2033 from USD 11.71 billion in 2023. This growth is attributed to increasing healthcare investments, expanding clinical trials, and a growing emphasis on personalized medicine in countries like China and India.North America Immune Checkpoint Inhibitors Market Report:
North America remains the largest market, expected to escalate from USD 22.55 billion in 2023 to USD 99.26 billion by 2033, driven by advanced healthcare systems, high disposable incomes, and a strong pipeline of novel therapies from leading organizations.South America Immune Checkpoint Inhibitors Market Report:
In South America, the market is set to rise from USD 0.68 billion in 2023 to USD 2.98 billion by 2033. The growth is stimulated by improving healthcare infrastructure, increasing awareness of cancer treatments, and a growing patient population.Middle East & Africa Immune Checkpoint Inhibitors Market Report:
In the Middle East and Africa, forecasted growth from USD 9.00 billion in 2023 to USD 39.63 billion by 2033 indicates increasing investment in healthcare and awareness of immune therapies, particularly in UAE and South Africa.Tell us your focus area and get a customized research report.
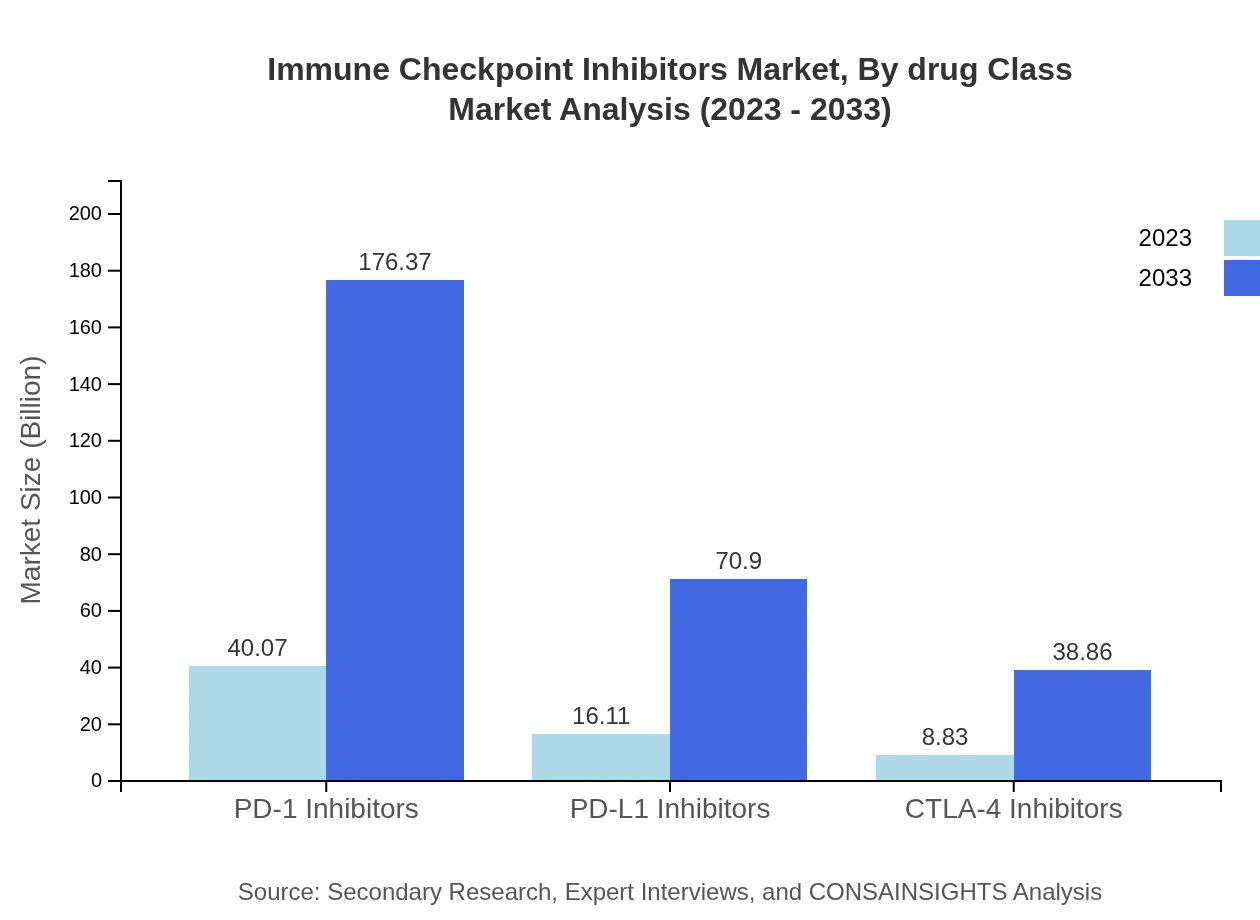
Immune Checkpoint Inhibitors Market Analysis By Drug Class
PD-1 inhibitors dominate the market, expected to reach USD 176.37 billion by 2033 from USD 40.07 billion in 2023, representing a CAGR of 14.3%. Meanwhile, PD-L1 inhibitors and CTLA-4 inhibitors follow with increasing shares as their effectiveness in combination therapies gains recognition.
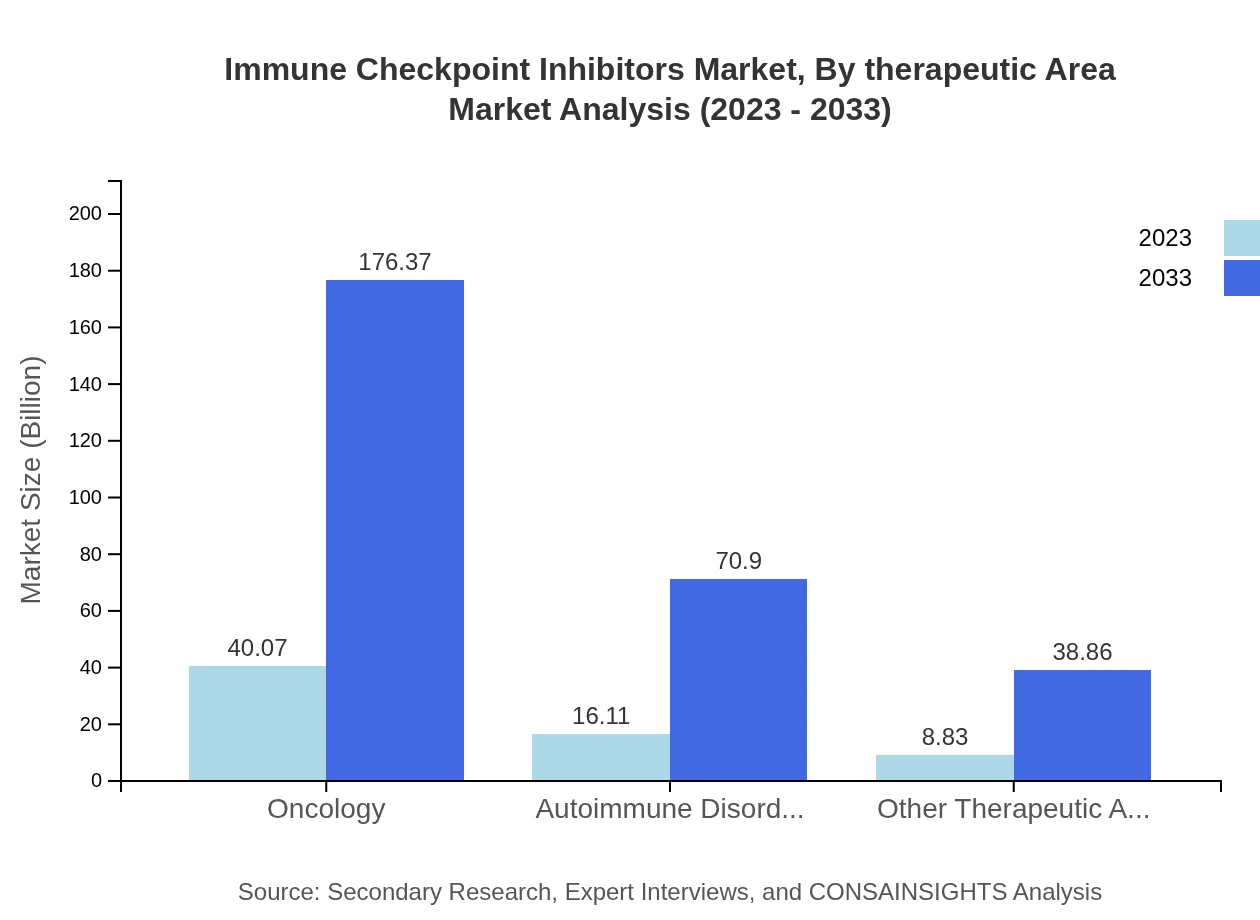
Immune Checkpoint Inhibitors Market Analysis By Therapeutic Area
The oncology segment, accounting for a significant portion of the market, is projected to grow from USD 40.07 billion in 2023 to USD 176.37 billion by 2033. Autoimmune disorders are also showing promise, with expected growth from USD 16.11 billion to USD 70.90 billion in the same time frame.
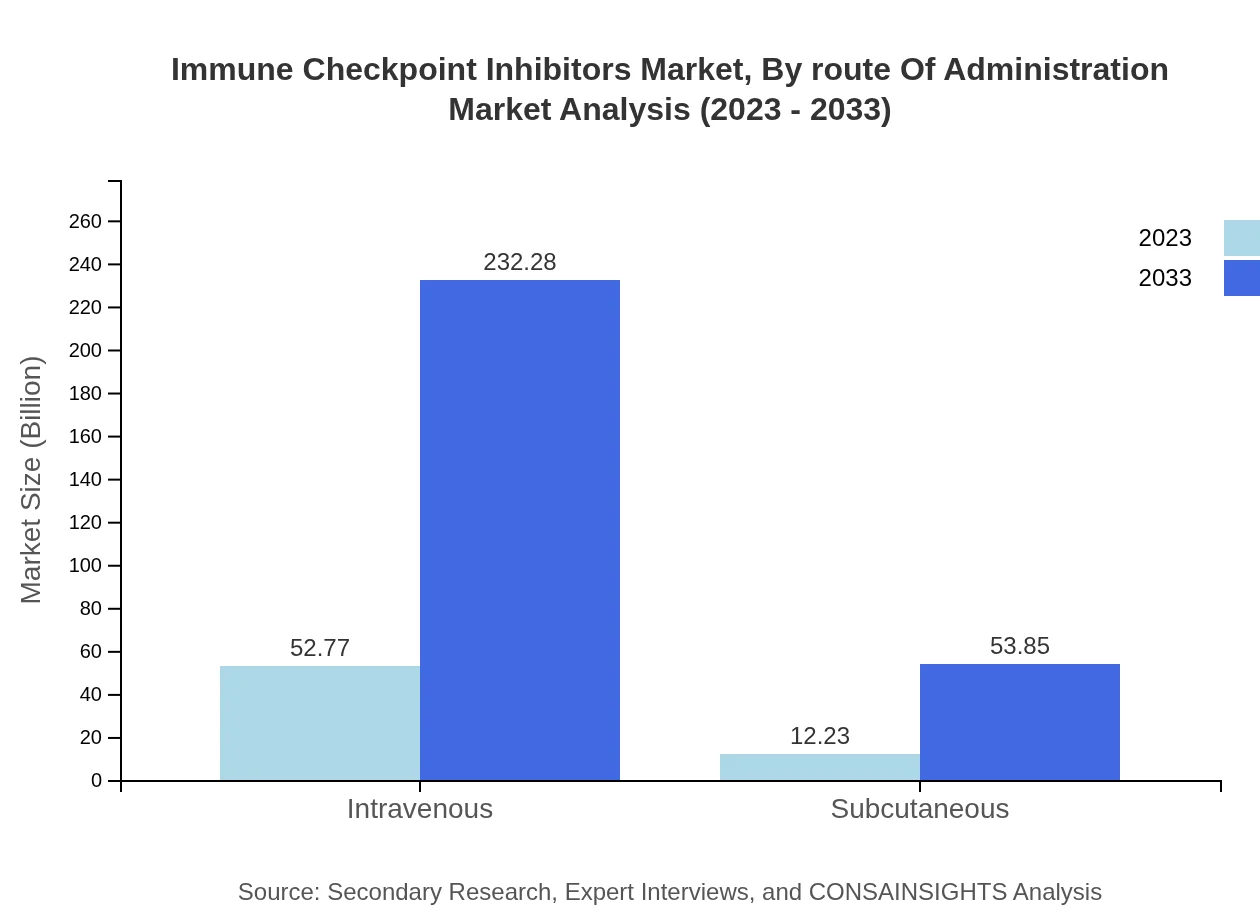
Immune Checkpoint Inhibitors Market Analysis By Route Of Administration
Intravenous administration leads the market, with an anticipated rise from USD 52.77 billion in 2023 to USD 232.28 billion by 2033, while subcutaneous methods are also gaining traction, expected to expand from USD 12.23 billion to USD 53.85 billion.
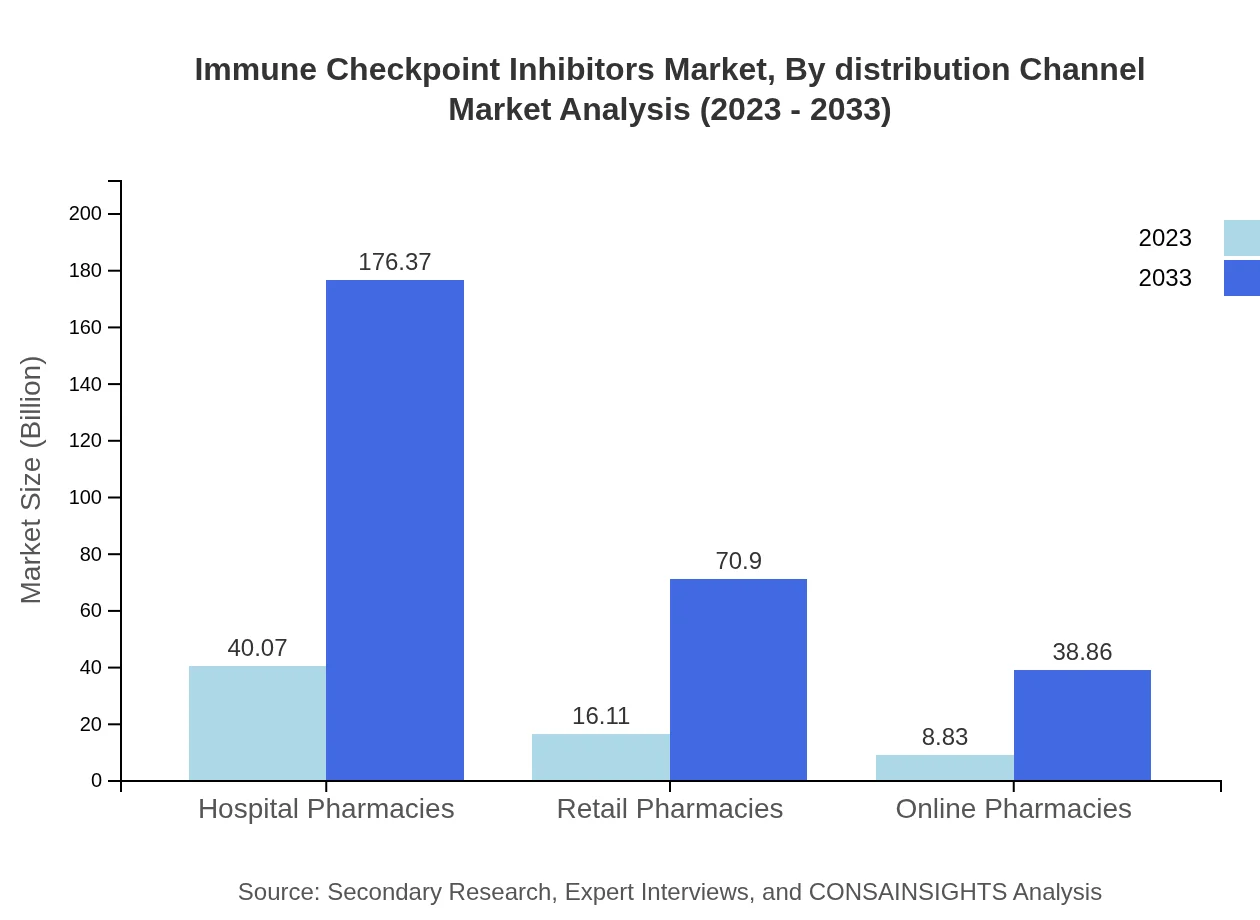
Immune Checkpoint Inhibitors Market Analysis By Distribution Channel
Hospital pharmacies comprise the largest distribution channel, projecting growth from USD 40.07 billion to USD 176.37 billion by 2033. Online pharmacies, while smaller, are increasing in relevance due to e-commerce trends in healthcare.
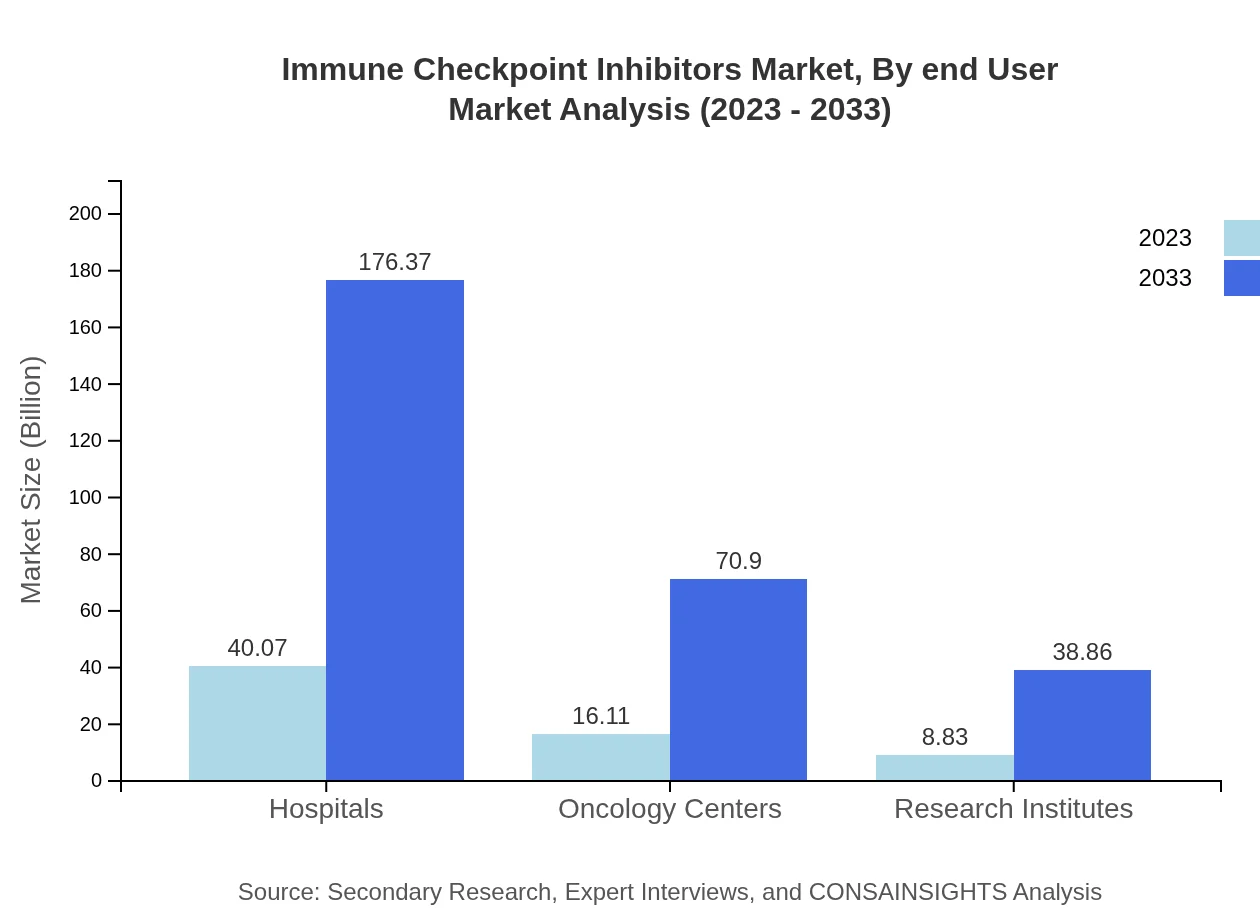
Immune Checkpoint Inhibitors Market Analysis By End User
The primary end-users are hospitals, indicating stability with growth from USD 40.07 billion in 2023 to USD 176.37 billion by 2033. Research institutes are also vital, expanding their roles in treatment development, from USD 8.83 billion to USD 38.86 billion.
Immune Checkpoint Inhibitors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Immune Checkpoint Inhibitors Industry
Bristol-Myers Squibb:
A pioneer in the field of immuno-oncology, known for its PD-1 inhibitor, Opdivo, and strong pipeline in cancer therapies.Merck & Co.:
A global leader in pharmaceuticals, noted for its Keytruda offering that has transformed therapy for multiple types of cancer.Roche:
Innovative in immunotherapy, particularly with its PD-L1 inhibitors and investment in combination therapies.AstraZeneca:
A significant player focusing on novel therapeutic areas with a strong commitment to oncology.Pfizer :
Key contributor in the field with several successful products in the checkpoint inhibitor space.We're grateful to work with incredible clients.









FAQs
What is the market size of immune Checkpoint Inhibitors?
The global immune checkpoint inhibitors market is anticipated to reach approximately $65 billion by 2033, growing at a compound annual growth rate (CAGR) of 15.2% from 2023, underlining significant expansion in the oncology segment.
What are the key market players or companies in this immune Checkpoint Inhibitors industry?
Key market players in the immune checkpoint inhibitors industry include major pharmaceutical companies like Bristol-Myers Squibb, Merck & Co., Roche, Novartis, and Pfizer. Their ongoing research and development efforts significantly contribute to market evolution.
What are the primary factors driving the growth in the immune Checkpoint Inhibitors industry?
Factors driving growth include increasing cancer prevalence, advances in medical technology, significant investments in research, favorable government regulations, and rising awareness of immunotherapy benefits, leading to greater adoption of immune checkpoint inhibitors.
Which region is the fastest Growing in the immune Checkpoint Inhibitors?
North America is poised to be the fastest-growing region, projected to grow from $22.55 billion in 2023 to $99.26 billion by 2033. Europe and Asia Pacific also show strong growth trajectories in immune checkpoint inhibitors.
Does ConsaInsights provide customized market report data for the immune Checkpoint Inhibitors industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the immune checkpoint inhibitors industry, helping stakeholders make informed decisions with in-depth insights and market analytics.
What deliverables can I expect from this immune Checkpoint Inhibitors market research project?
Deliverables include comprehensive market reports, insights into key trends, segmentation data, competitive analysis, regional forecasts, and actionable recommendations to support strategic planning in the immune checkpoint inhibitors market.
What are the market trends of immune Checkpoint Inhibitors?
Current trends include an increasing focus on combination therapies, advancements in personalized medicine, rising demand for monoclonal antibodies, and heightened exploration of immune checkpoint inhibitors for various cancers and autoimmune disorders.