Immuno Oncology Assays Market Report
Published Date: 31 January 2026 | Report Code: immuno-oncology-assays
Immuno Oncology Assays Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Immuno Oncology Assays market from 2023 to 2033, offering insights into market size, trends, segmentation, regional analysis, and competitive landscape. It aims to inform stakeholders about the key factors influencing market growth and future opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
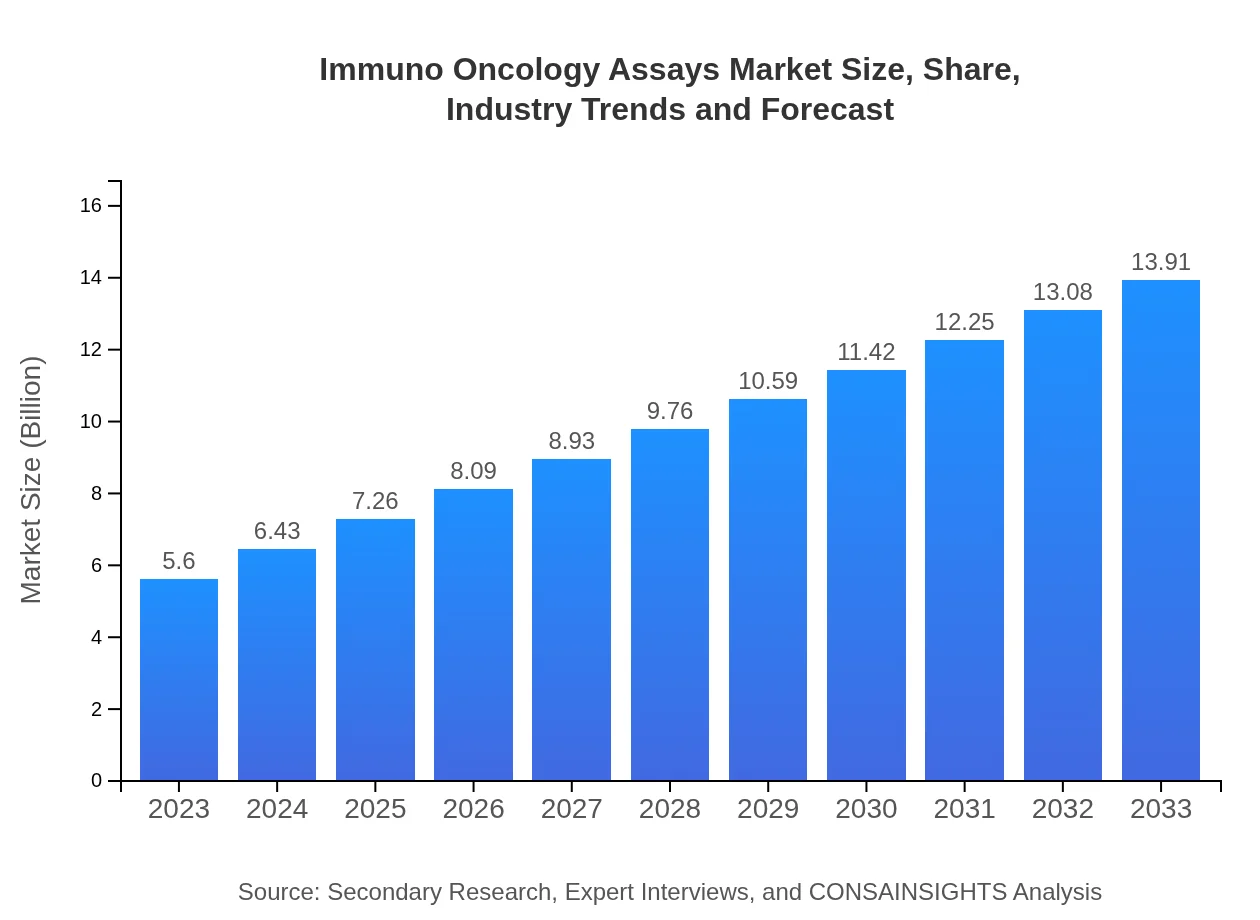
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $13.91 Billion |
| Top Companies | Roche, Thermo Fisher Scientific, Agilent Technologies, BD Biosciences |
| Last Modified Date | 31 January 2026 |
Immuno Oncology Assays Market Overview
Customize Immuno Oncology Assays Market Report market research report
- ✔ Get in-depth analysis of Immuno Oncology Assays market size, growth, and forecasts.
- ✔ Understand Immuno Oncology Assays's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Immuno Oncology Assays
What is the Market Size & CAGR of Immuno Oncology Assays market in 2023?
Immuno Oncology Assays Industry Analysis
Immuno Oncology Assays Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Immuno Oncology Assays Market Analysis Report by Region
Europe Immuno Oncology Assays Market Report:
In Europe, the market for Immuno Oncology Assays is set to grow from $1.75 billion in 2023 to $4.35 billion by 2033, driven by increasing cancer incidences and supportive regulatory frameworks enhancing market access for innovative assays.Asia Pacific Immuno Oncology Assays Market Report:
The Asia Pacific region is anticipated to witness significant growth in the Immuno Oncology Assays market, with a projected market value of $2.34 billion by 2033, up from $0.94 billion in 2023. This growth is driven by rising healthcare expenditure and increasing awareness about cancer diagnostics.North America Immuno Oncology Assays Market Report:
North America holds the largest share of the Immuno Oncology Assays market, valued at $2.15 billion in 2023 and anticipated to reach $5.33 billion by 2033. The region's advanced healthcare systems and significant investments in cancer research are major contributors.South America Immuno Oncology Assays Market Report:
In South America, the Immuno Oncology Assays market is expected to grow from $0.55 billion in 2023 to $1.36 billion by 2033. The expanding healthcare infrastructure and collaborations with global pharmaceutical firms are likely to accelerate this growth.Middle East & Africa Immuno Oncology Assays Market Report:
The Middle East and Africa market, though smaller, is expected to grow from $0.21 billion in 2023 to $0.53 billion by 2033. This growth is spurred by improving healthcare facilities and an increasing focus on cancer research.Tell us your focus area and get a customized research report.
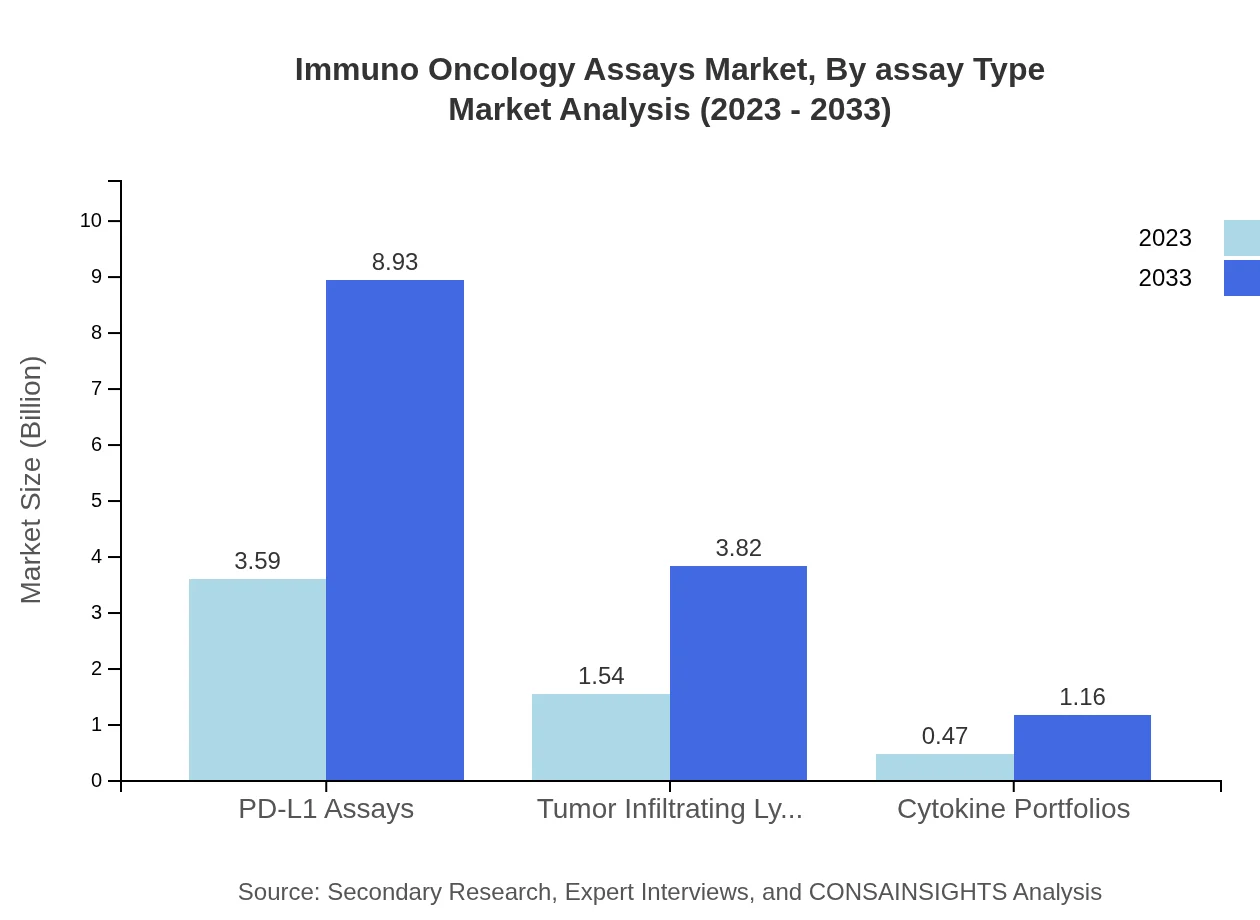
Immuno Oncology Assays Market Analysis By Assay Type
In the by assay type segment, PD-L1 assays lead the market with a size of $3.59 billion in 2023 and expected to reach $8.93 billion by 2033, maintaining a substantial market share of 64.19% throughout the forecast period. Other notable assays include tumor infiltrating lymphocytes and cytokine assays, which play important roles in both therapeutic and diagnostic applications.
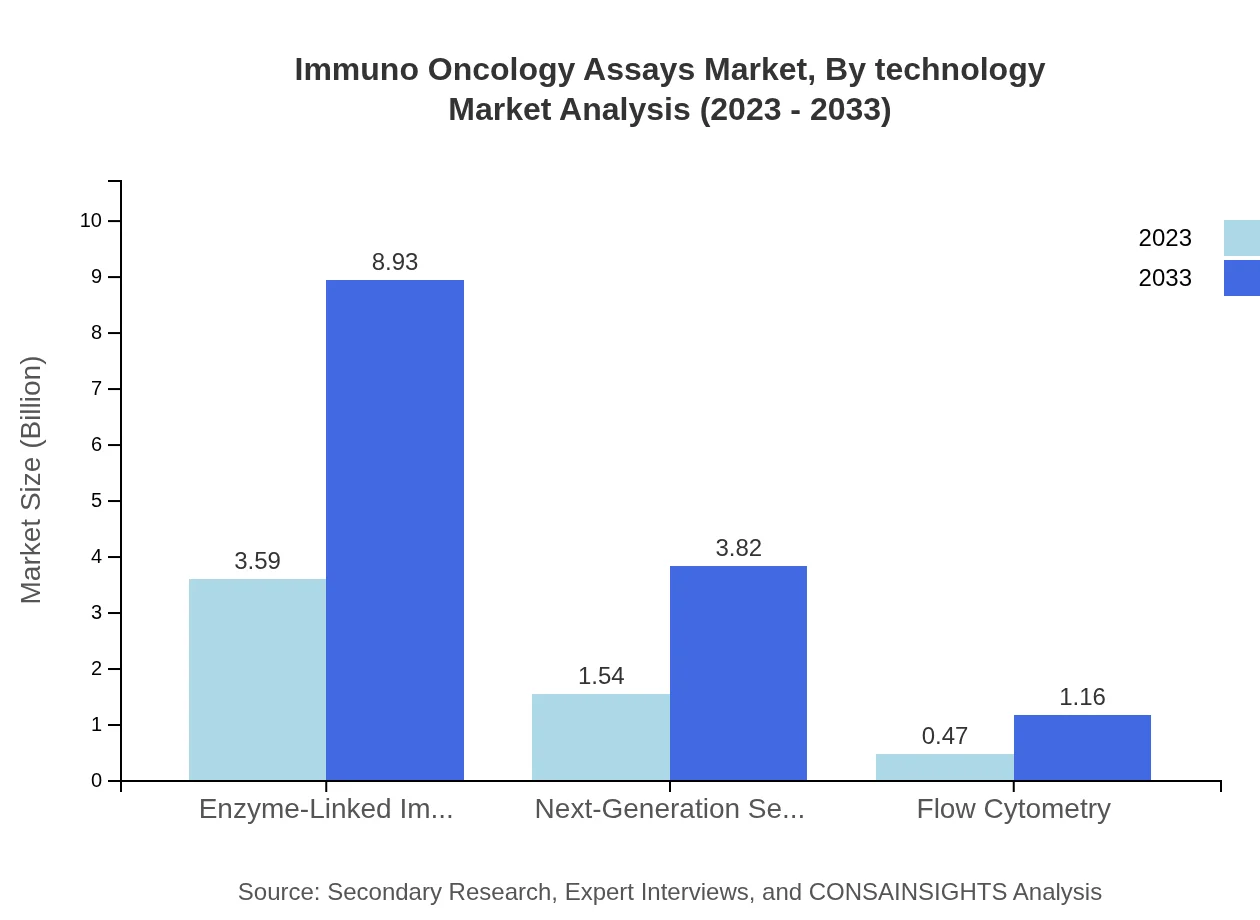
Immuno Oncology Assays Market Analysis By Technology
The technology segment shows promising growth, with ELISA leading at $3.59 billion in 2023. Additionally, advanced technologies such as NGS are carving out their space with an expected increase from $1.54 billion in 2023 to $3.82 billion by 2033.
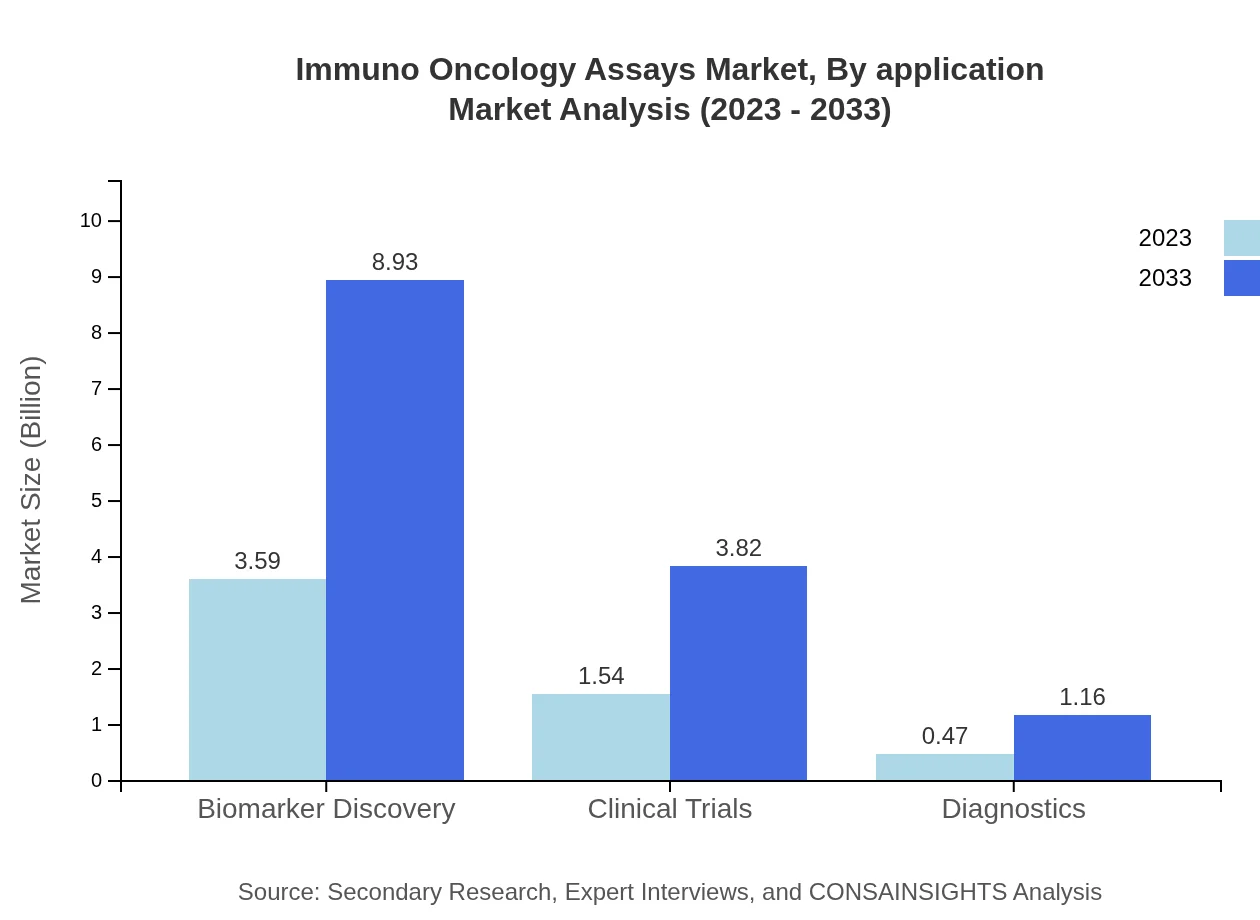
Immuno Oncology Assays Market Analysis By Application
Applications in clinical trials are critical, with the segment size anticipated to grow significantly from $1.54 billion in 2023 to $3.82 billion by 2033, spurred by partnerships between bio-pharmaceutical firms and clinical research organizations.
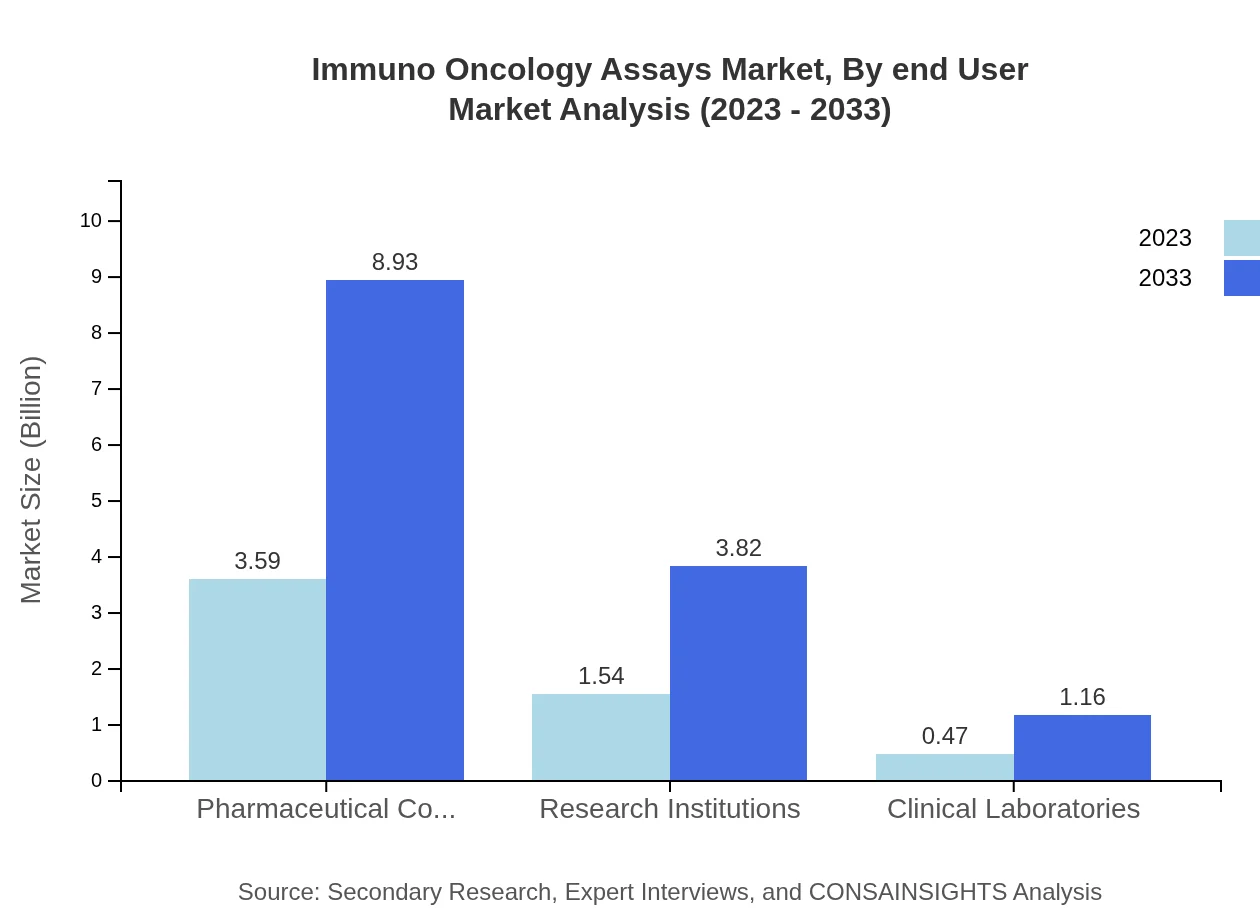
Immuno Oncology Assays Market Analysis By End User
Key end-users include pharmaceutical companies, which accounted for over 64% market share in 2023 with a size of $3.59 billion, reflecting their pivotal role in driving assay demand for drug development and screening.
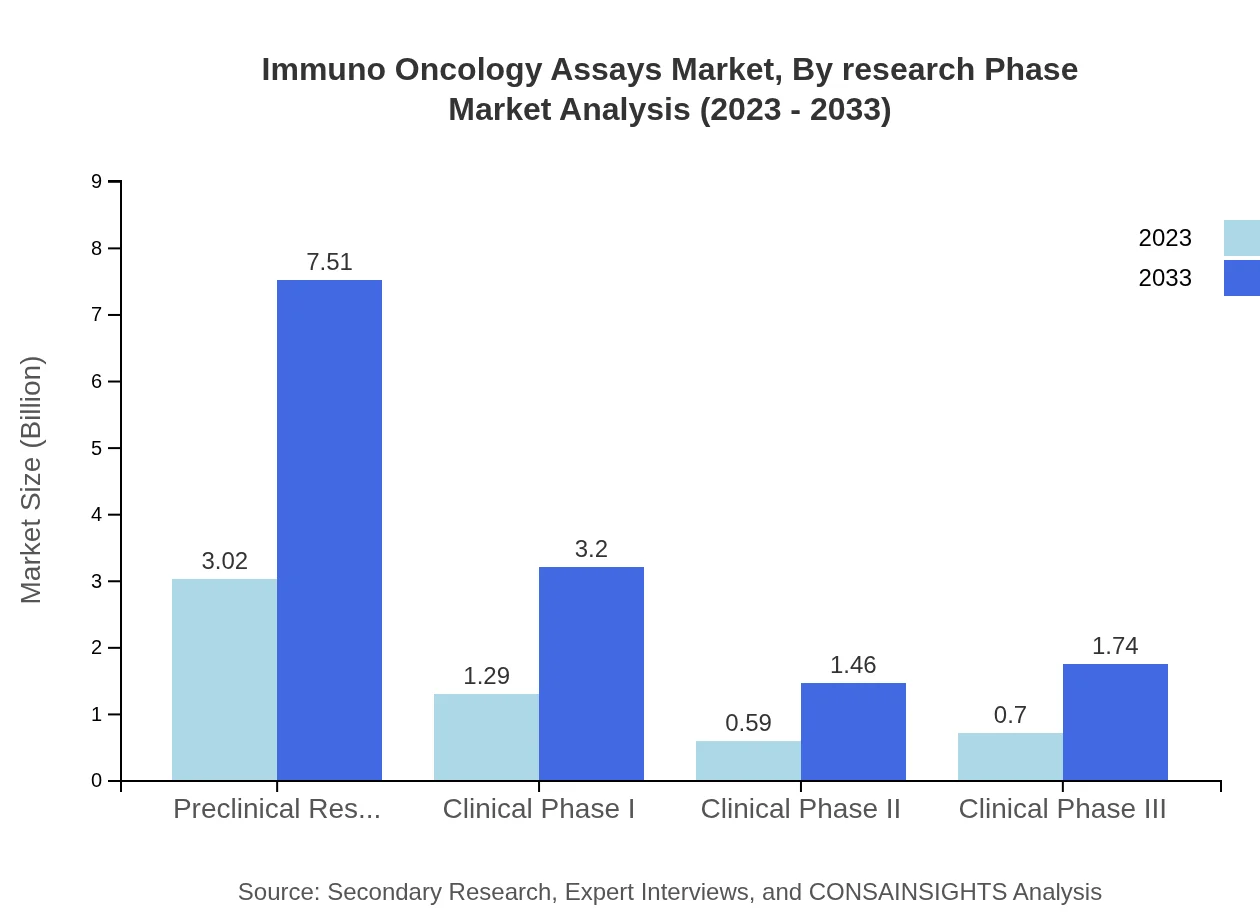
Immuno Oncology Assays Market Analysis By Research Phase
The market is segmented based on research phases, with preclinical research dominating at $3.02 billion in 2023, expected to reach $7.51 billion by 2033, indicating the increasing emphasis on early-stage discovery.
Immuno Oncology Assays Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Immuno Oncology Assays Industry
Roche:
A leader in diagnostic and pharmaceutical products, Roche has developed several benchmark immunoassays that advance the field of personalized medicine in oncology.Thermo Fisher Scientific:
Known for their vast portfolio of assay technologies, Thermo Fisher provides critical immuno-oncology solutions and analytical services to healthcare providers.Agilent Technologies:
Agilent is a significant player in biomarker research, providing cutting-edge assays and analytics that enhance drug discovery in oncology.BD Biosciences:
BD offers a range of solutions for researchers in immuno-oncology, including flow cytometry assays that are essential for tumor profiling.We're grateful to work with incredible clients.









FAQs
What is the market size of immuno Oncology Assays?
As of 2023, the immuno-oncology assays market is valued at 5.6 billion USD, with a projected CAGR of 9.2% through 2033. This growth reflects the increasing demand for innovative cancer therapies and diagnostic methods.
What are the key market players or companies in the immuno Oncology Assays industry?
The key players in the immuno-oncology assays market include renowned pharmaceutical companies, biotech firms, and specialized diagnostic laboratories that focus on developing and commercializing advanced assays for cancer treatment and monitoring.
What are the primary factors driving the growth in the immuno Oncology Assays industry?
Driven by rising cancer incidence, increased R&D investment, and regulatory support for personalized medicine, the immuno-oncology assays industry is experiencing significant growth, facilitating better patient outcomes through tailored cancer therapies.
Which region is the fastest Growing in the immuno Oncology Assays?
North America is the fastest-growing region in the immuno-oncology assays market, projecting an increase from 2.15 billion USD in 2023 to 5.33 billion USD by 2033, driven by innovation and investment in cancer research.
Does ConsaInsights provide customized market report data for the immuno Oncology Assays industry?
Yes, ConsaInsights offers customized market report data tailored to specific inquiries and needs within the immuno-oncology assays industry, ensuring clients receive relevant and actionable insights for informed decision-making.
What deliverables can I expect from this immuno Oncology Assays market research project?
Expect comprehensive reports detailing market size, growth projections, competitive landscape analysis, segment data, and critical trends, all designed to enhance strategic planning for stakeholders in the immuno-oncology assays market.
What are the market trends of immuno Oncology Assays?
Emerging trends in the immuno-oncology assays market include advancements in biomarker identification, growing adoption of Next-Generation Sequencing (NGS), and increasing focus on companion diagnostics to improve treatment efficacy.