Immunoprotein Diagnostic Testing Market Report
Published Date: 31 January 2026 | Report Code: immunoprotein-diagnostic-testing
Immunoprotein Diagnostic Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Immunoprotein Diagnostic Testing market, encompassing market size, growth trends, and regional insights from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
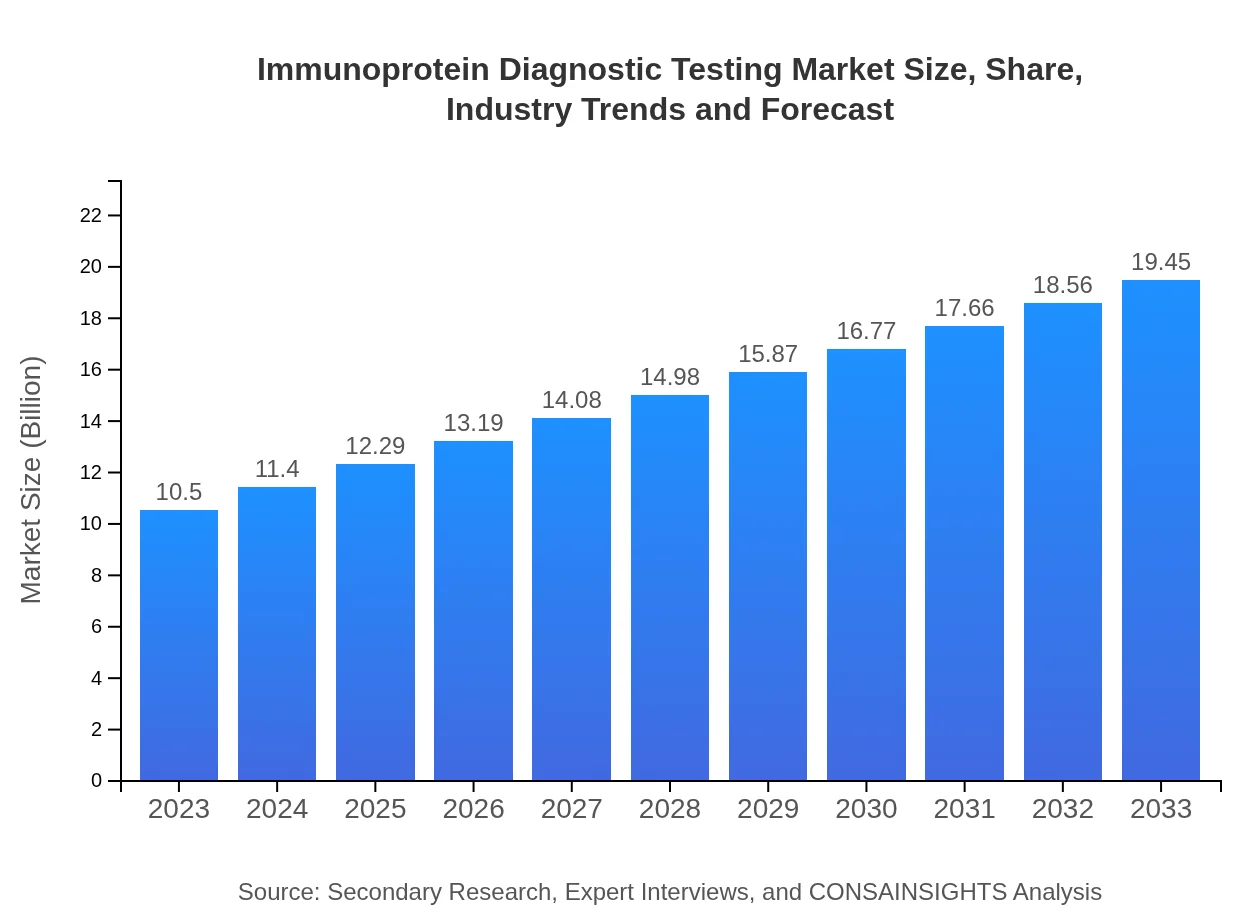
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $19.45 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Immunoprotein Diagnostic Testing Market Overview
Customize Immunoprotein Diagnostic Testing Market Report market research report
- ✔ Get in-depth analysis of Immunoprotein Diagnostic Testing market size, growth, and forecasts.
- ✔ Understand Immunoprotein Diagnostic Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Immunoprotein Diagnostic Testing
What is the Market Size & CAGR of Immunoprotein Diagnostic Testing market in 2023?
Immunoprotein Diagnostic Testing Industry Analysis
Immunoprotein Diagnostic Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
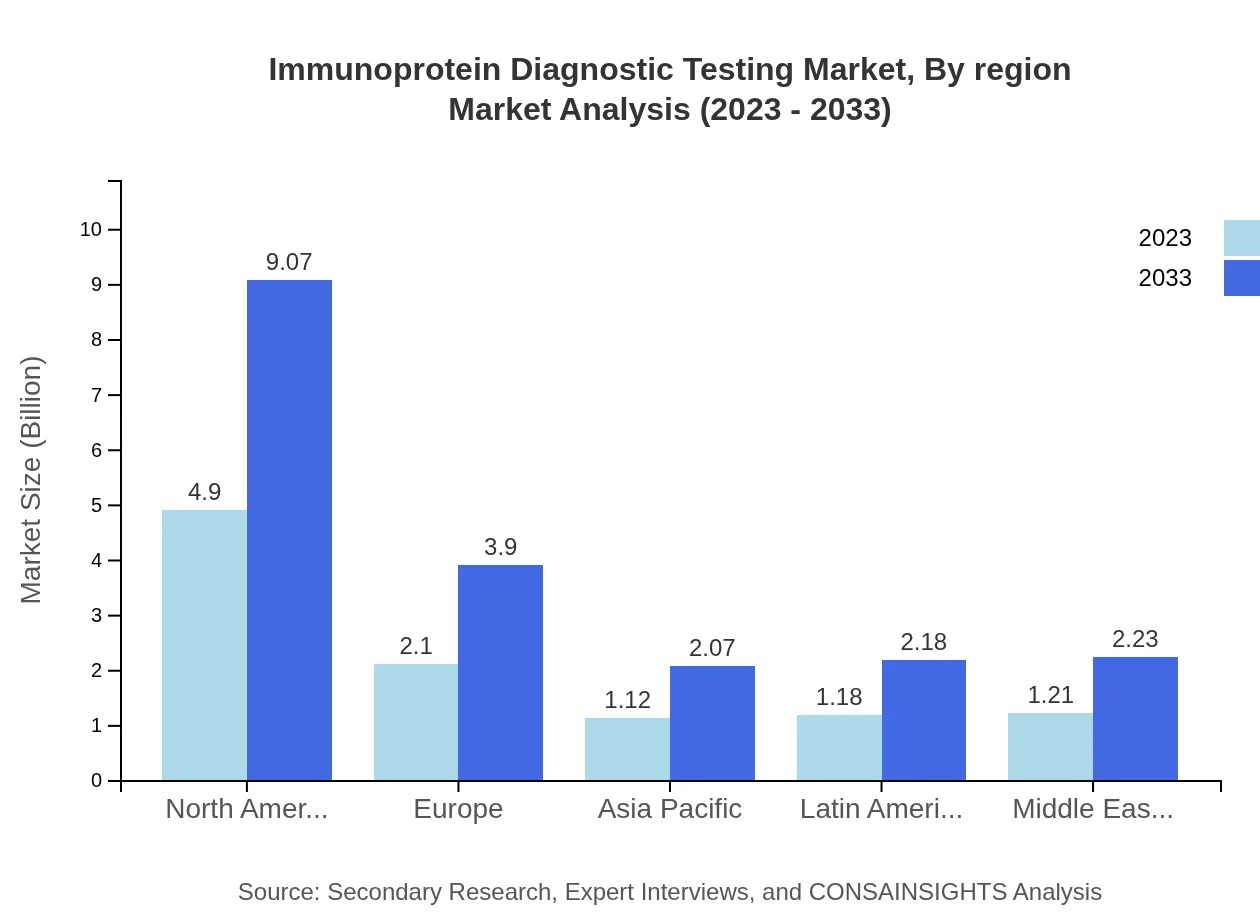
Immunoprotein Diagnostic Testing Market Analysis Report by Region
Europe Immunoprotein Diagnostic Testing Market Report:
In Europe, the market is set to grow from USD 2.56 billion in 2023 to USD 4.75 billion by 2033, supported by the increasing adoption of advanced immunodiagnostic tests and favorable reimbursement policies.Asia Pacific Immunoprotein Diagnostic Testing Market Report:
In the Asia Pacific region, the Immunoprotein Diagnostic Testing market is expected to grow from USD 2.28 billion in 2023 to USD 4.23 billion by 2033. Key contributing factors include increased healthcare expenditure, rising incidences of chronic diseases, and improvements in diagnostic facilities.North America Immunoprotein Diagnostic Testing Market Report:
North America dominated the market with a valuation of USD 4.03 billion in 2023, expected to achieve USD 7.47 billion by 2033. This region benefits from a robust healthcare infrastructure, strong R&D activities, and a high prevalence of autoimmune conditions.South America Immunoprotein Diagnostic Testing Market Report:
The South American market is projected to expand from USD 1.04 billion in 2023 to USD 1.92 billion by 2033, driven by the growing demand for advanced diagnostic solutions and increased investments in the healthcare sector.Middle East & Africa Immunoprotein Diagnostic Testing Market Report:
The Middle East and Africa market is expected to expand from USD 0.58 billion in 2023 to USD 1.08 billion by 2033, influenced by rising healthcare awareness and the introduction of new diagnostic technologies.Tell us your focus area and get a customized research report.
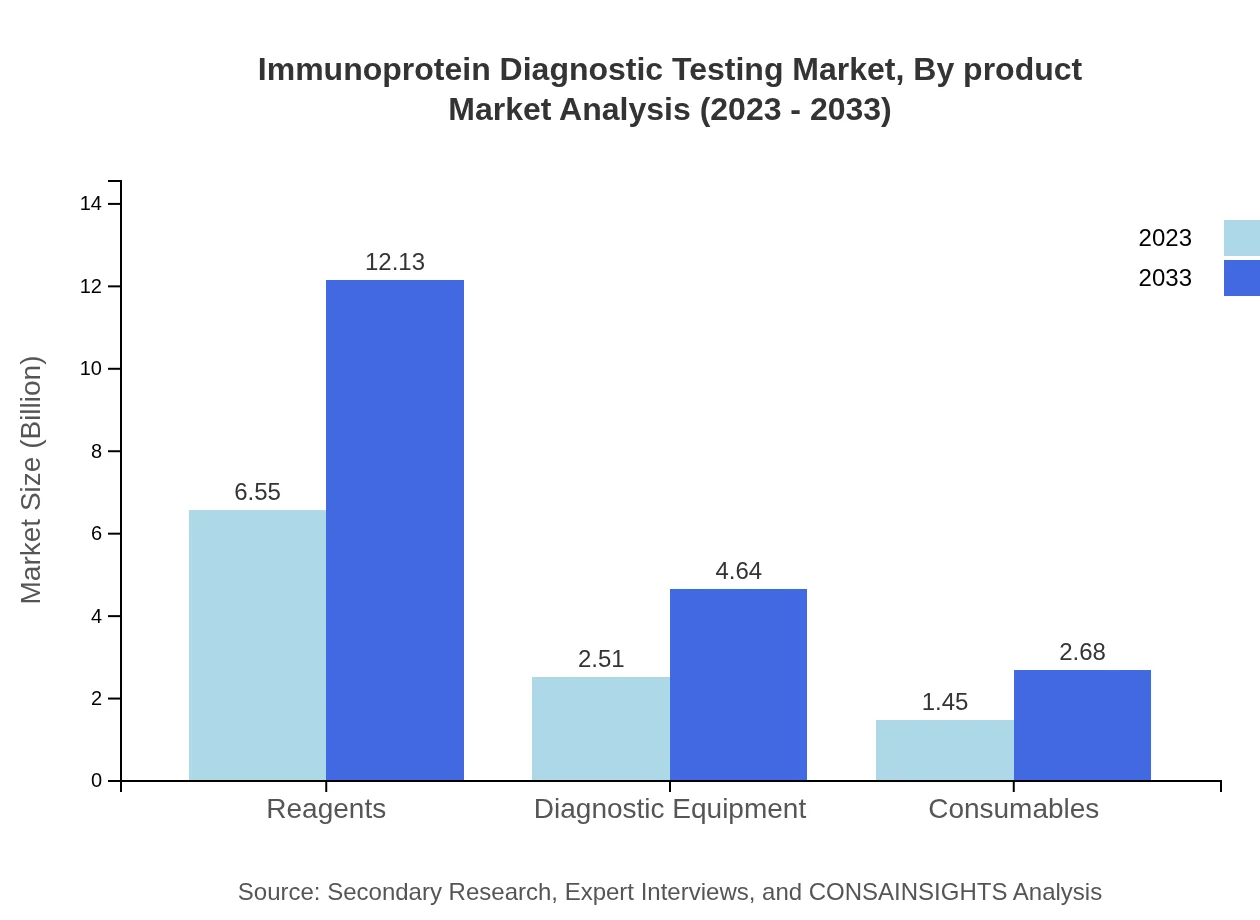
Immunoprotein Diagnostic Testing Market Analysis By Product
Reagents hold the largest market share, valued at USD 6.55 billion in 2023 and projected to reach USD 12.13 billion by 2033, accounting for 62.35% of the market. Diagnostic equipment and consumables are also significant segments, with respective market sizes of USD 2.51 billion and USD 1.45 billion.
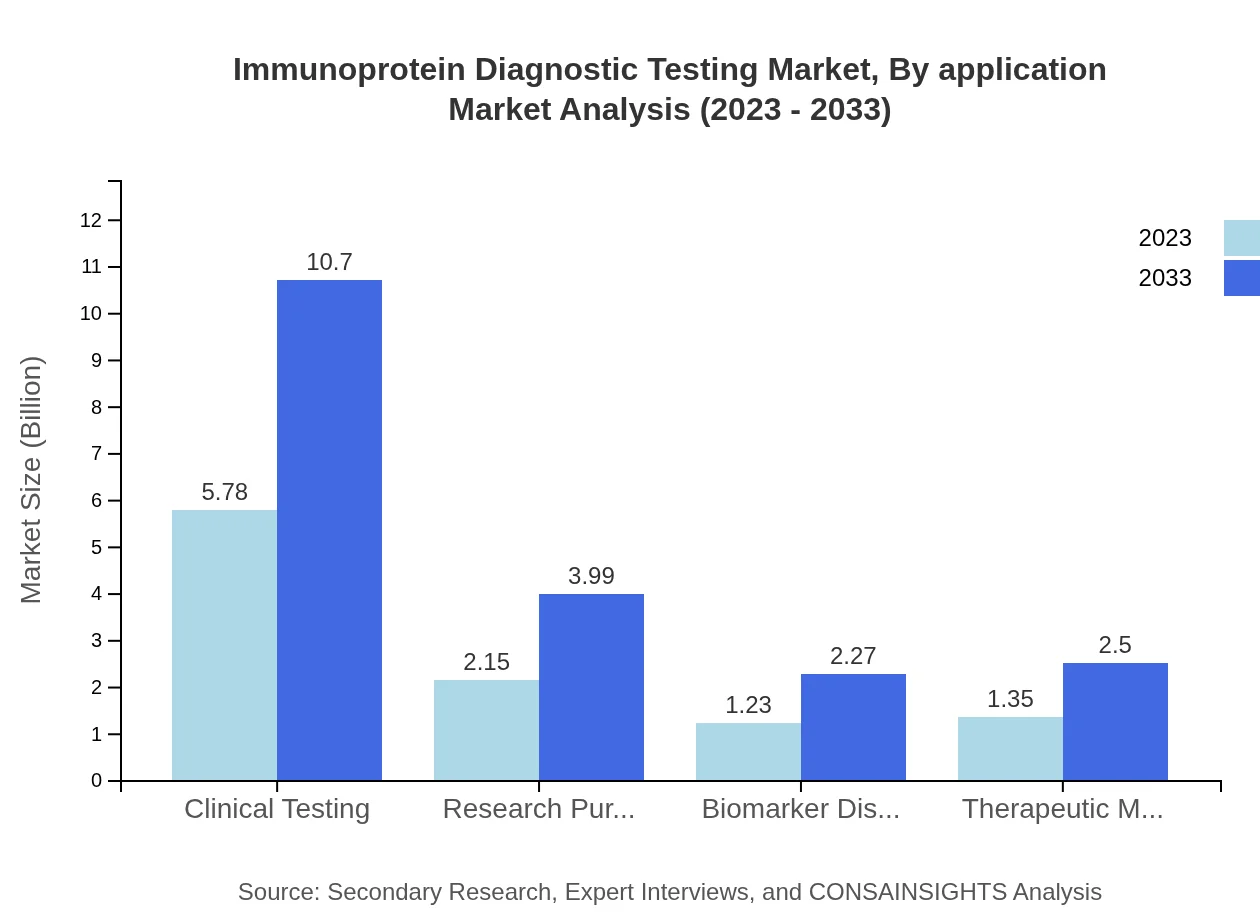
Immunoprotein Diagnostic Testing Market Analysis By Application
Clinical testing remains the leading application area, constituting a major share of the market at 55%. The segment is expected to grow from USD 5.78 billion in 2023 to USD 10.70 billion by 2033. Other notable segments include research purposes and therapeutic monitoring, with significant implications for drug development and patient management.
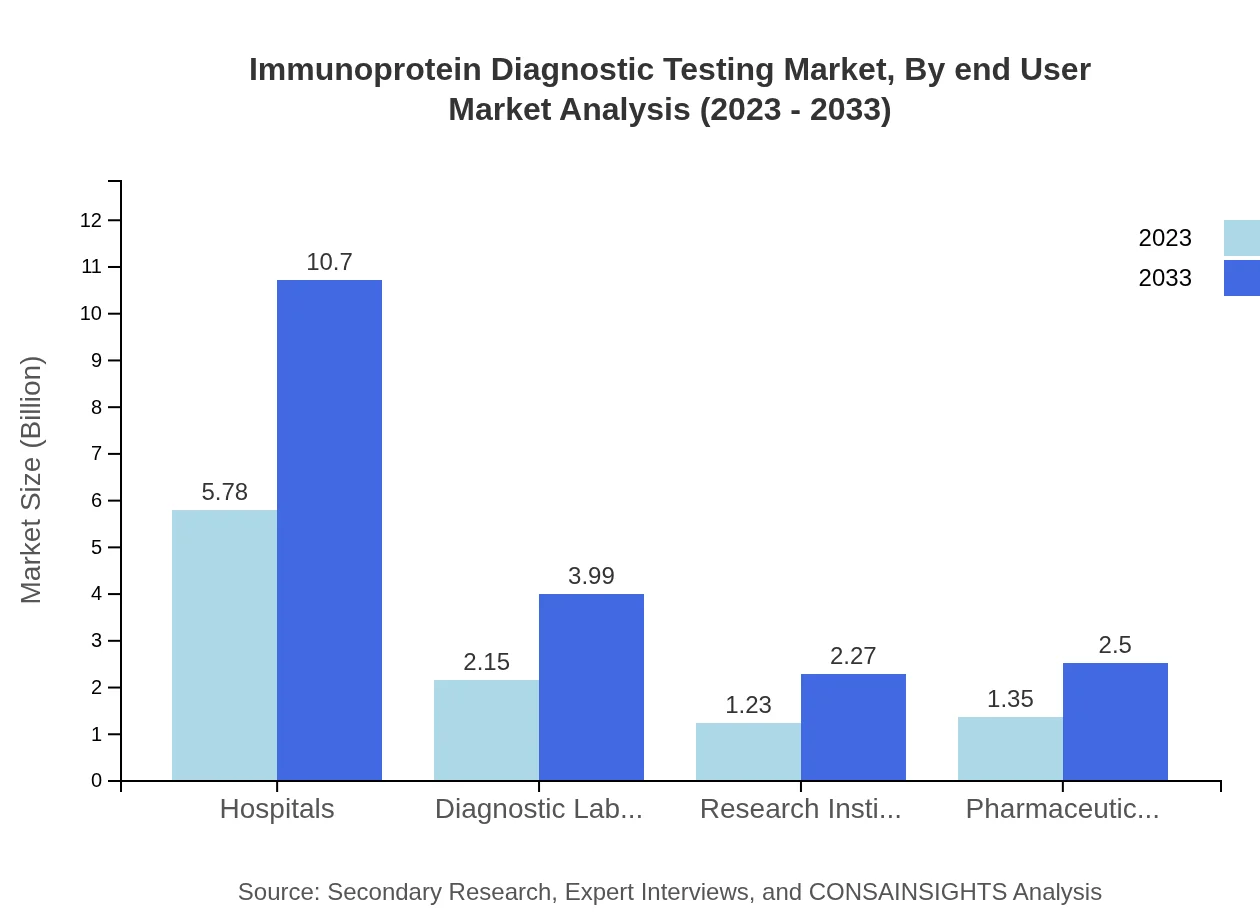
Immunoprotein Diagnostic Testing Market Analysis By End User
Hospitals represent the primary end-user of Immunoprotein Diagnostic Testing services, with a market size of USD 5.78 billion in 2023. Research institutes and pharmaceutical companies are also key participants, leveraging diagnostic tests for drug efficacy and clinical studies.
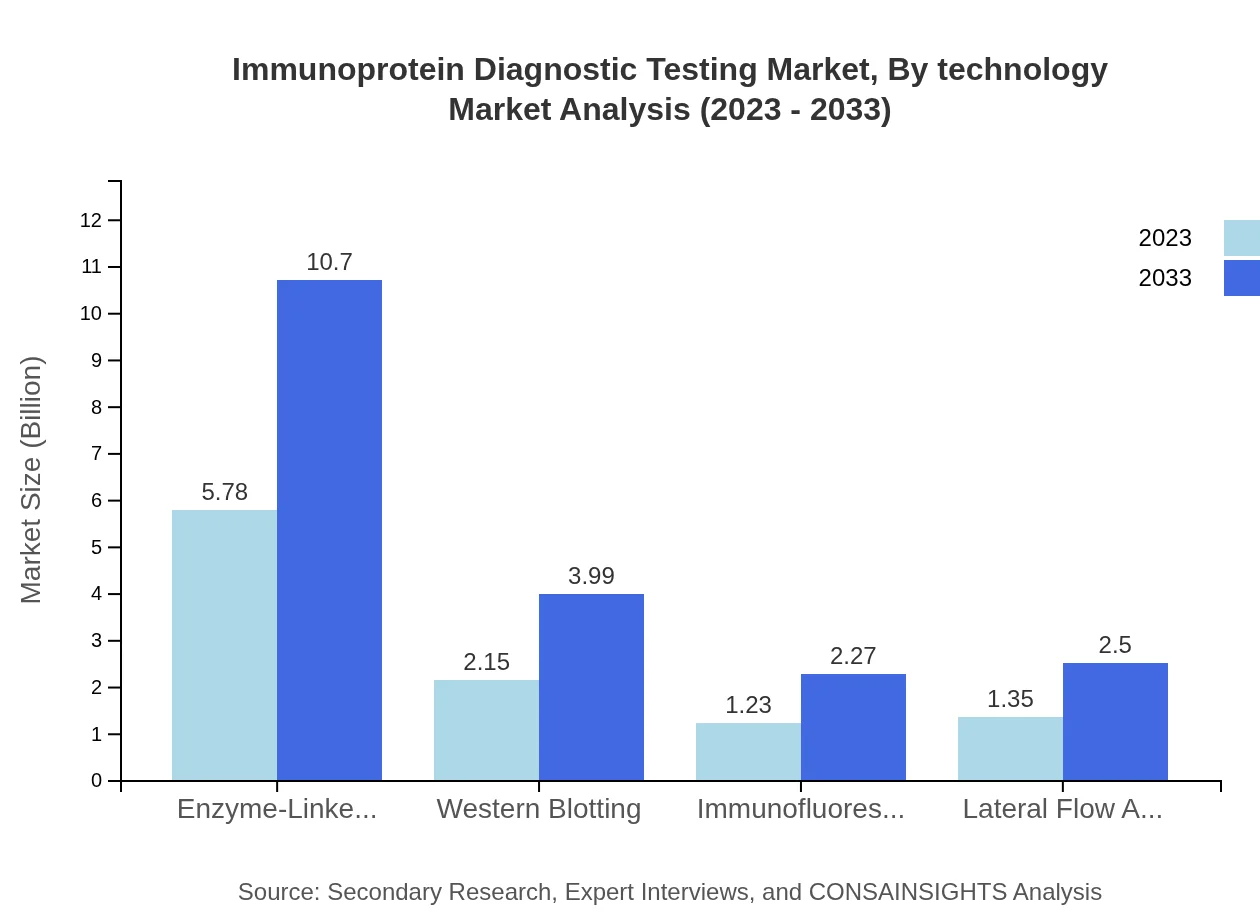
Immunoprotein Diagnostic Testing Market Analysis By Technology
The Enzyme-Linked Immunosorbent Assay (ELISA) methodology is dominant, accounting for USD 5.78 billion in 2023 and maintaining a share of 55%. Other technologies such as Western blotting and lateral flow assays also contribute significantly to market dynamics.
Immunoprotein Diagnostic Testing Market Analysis By Region
Regional analysis highlights North America as the largest market for Immunoprotein Diagnostics, followed by Europe and Asia Pacific, indicating diverse growth opportunities depending on local healthcare systems and trends.
Immunoprotein Diagnostic Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Immunoprotein Diagnostic Testing Industry
Roche Diagnostics:
A leader in innovative IVD solutions, Roche Diagnostics provides a wide range of immunodiagnostic tests known for their accuracy and reliability.Abbott Laboratories:
Abbott manufactures diagnostic tests and devices, including advanced immunoassays that facilitate effective patient monitoring and disease management.Thermo Fisher Scientific:
This company specializes in providing laboratory equipment, reagents, and diagnostic tests essential for immunoprotein diagnostics and research.Siemens Healthineers:
Siemens plays a critical role in diagnostic imaging and laboratory diagnostics, emphasizing innovative immunoproducts that enhance diagnostic accuracy.We're grateful to work with incredible clients.









FAQs
What is the market size of immunoprotein Diagnostic Testing?
The immunoprotein diagnostic testing market is projected to reach a size of approximately $10.5 billion by 2033, growing from a baseline value in 2023, with a robust compound annual growth rate (CAGR) of 6.2% anticipated during this period.
What are the key market players or companies in this immunoprotein Diagnostic Testing industry?
Key players in the immunoprotein diagnostic testing industry include major companies such as Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific, which continue to innovate and expand their market reach.
What are the primary factors driving the growth in the immunoprotein Diagnostic Testing industry?
The growth drivers for the immunoprotein diagnostic testing market include advancements in diagnostic technologies, increasing prevalence of chronic diseases, and the rising demand for early and accurate disease diagnostics, which enhance patient outcomes.
Which region is the fastest Growing in the immunoprotein Diagnostic Testing?
The fastest-growing region in the immunoprotein diagnostic testing market is North America, projected to expand significantly from approximately $4.03 billion in 2023 to $7.47 billion by 2033, representing a strong demand for diagnostic solutions.
Does ConsaInsights provide customized market report data for the immunoprotein Diagnostic Testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific client requirements in the immunoprotein diagnostic testing industry, ensuring that stakeholders receive relevant and actionable insights for informed decision-making.
What deliverables can I expect from this immunoprotein Diagnostic Testing market research project?
Deliverables from the immunoprotein diagnostic testing market research project typically include detailed market analysis, segmentation data, competitive landscape assessments, and actionable insights specifically designed to guide business strategies and investments.
What are the market trends of immunoprotein Diagnostic Testing?
Current trends in the immunoprotein diagnostic testing market include the increasing utilization of point-of-care testing, advancements in biomarker identification, and the rising adoption of personalized medicine, which are shaping future market dynamics.