Immunotherapy Drugs Market Report
Published Date: 31 January 2026 | Report Code: immunotherapy-drugs
Immunotherapy Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Immunotherapy Drugs market, including key trends, market size, growth forecasts for the period 2023-2033, and segmentation across various categories. It offers critical insights into regional performance and global market leaders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
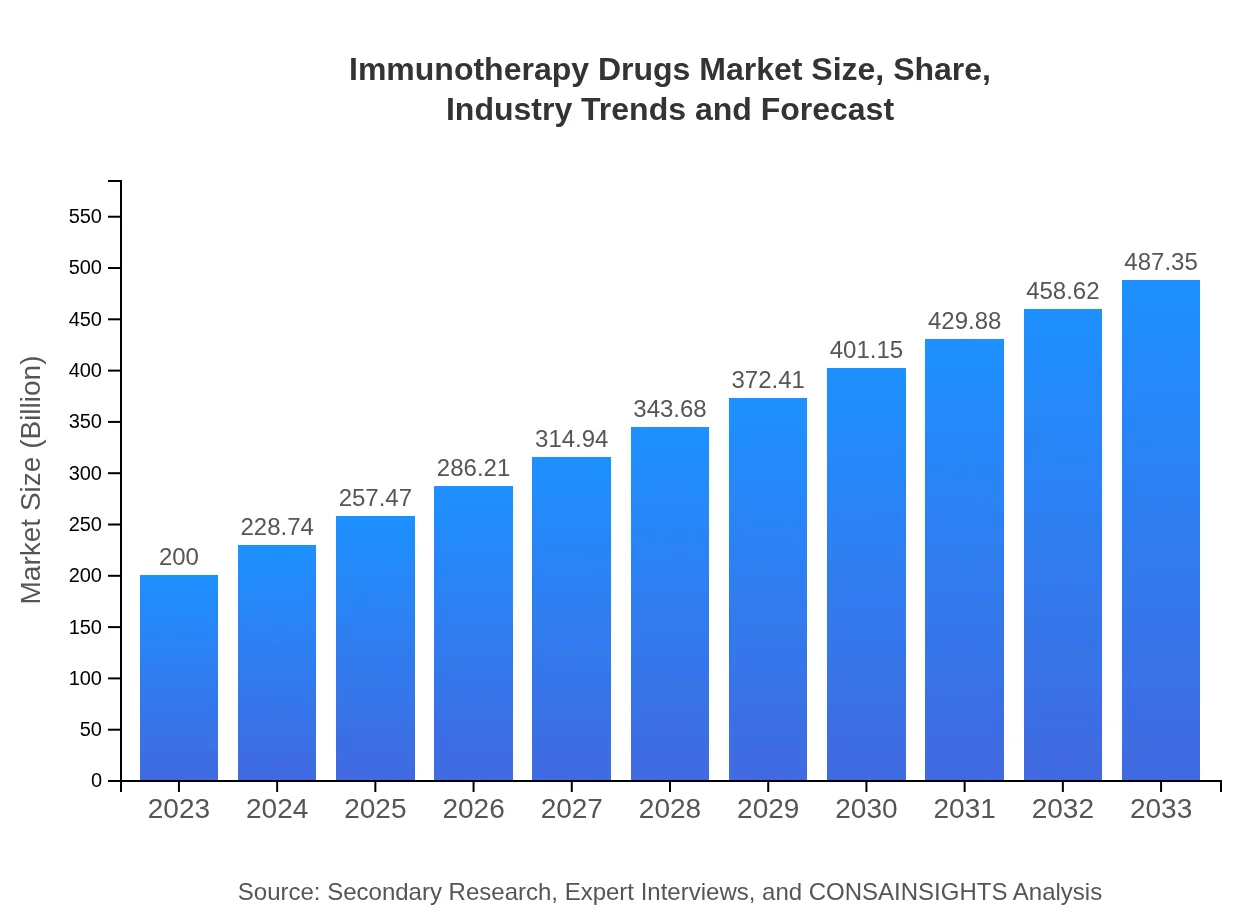
| 2023 Market Size | $200.00 Billion |
| CAGR (2023-2033) | 9% |
| 2033 Market Size | $487.35 Billion |
| Top Companies | Roche, Bristol Myers Squibb, Merck & Co., Pfizer , Novartis |
| Last Modified Date | 31 January 2026 |
Immunotherapy Drugs Market Overview
Customize Immunotherapy Drugs Market Report market research report
- ✔ Get in-depth analysis of Immunotherapy Drugs market size, growth, and forecasts.
- ✔ Understand Immunotherapy Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Immunotherapy Drugs
What is the Market Size & CAGR of Immunotherapy Drugs market in 2033?
Immunotherapy Drugs Industry Analysis
Immunotherapy Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Immunotherapy Drugs Market Analysis Report by Region
Europe Immunotherapy Drugs Market Report:
Europe's immunotherapy drugs market was valued at USD 60.56 billion in 2023, projected to grow to USD 147.57 billion by 2033. European countries are increasingly adopting immunotherapy due to robust research pipelines and patient-centric treatment approaches. The collaboration between academia and industry fosters innovation, while patient advocacy for advanced treatments influences market dynamics positively.Asia Pacific Immunotherapy Drugs Market Report:
In the Asia-Pacific region, the immunotherapy drugs market was valued at approximately USD 36.78 billion in 2023 and is projected to reach USD 89.62 billion by 2033. This region is witnessing a rapid increase in cancer prevalence, coupled with growing healthcare expenditures, which facilitate access to advanced immunotherapy treatments. The expanding pharmaceutical manufacturing base and partnerships for clinical trials are set to drive market growth.North America Immunotherapy Drugs Market Report:
North America held the largest market share, with a valuation of USD 73.58 billion in 2023, expected to reach USD 179.30 billion by 2033. The region benefits from established healthcare infrastructure, substantial R&D investments, and a high concentration of key players and innovators in the immunotherapy sector. Regulatory support for innovative therapies and reimbursement policies further enhance market potential.South America Immunotherapy Drugs Market Report:
South America's immunotherapy drugs market, valued at around USD 4.68 billion in 2023, is anticipated to grow to USD 11.40 billion by 2033. The growth is primarily attributed to increasing investment in healthcare and rising awareness about cancer treatment options. However, economic constraints and healthcare accessibility issues may pose challenges for sustainable growth.Middle East & Africa Immunotherapy Drugs Market Report:
The Middle East and Africa show gradual growth in the immunotherapy drugs market, with an estimated market size of USD 24.40 billion in 2023, expected to reach USD 59.46 billion by 2033. Investment in healthcare infrastructure, increasing disease burden, and governmental initiatives promoting cancer research are key growth drivers in this region.Tell us your focus area and get a customized research report.
Immunotherapy Drugs Market Analysis By Drug Type
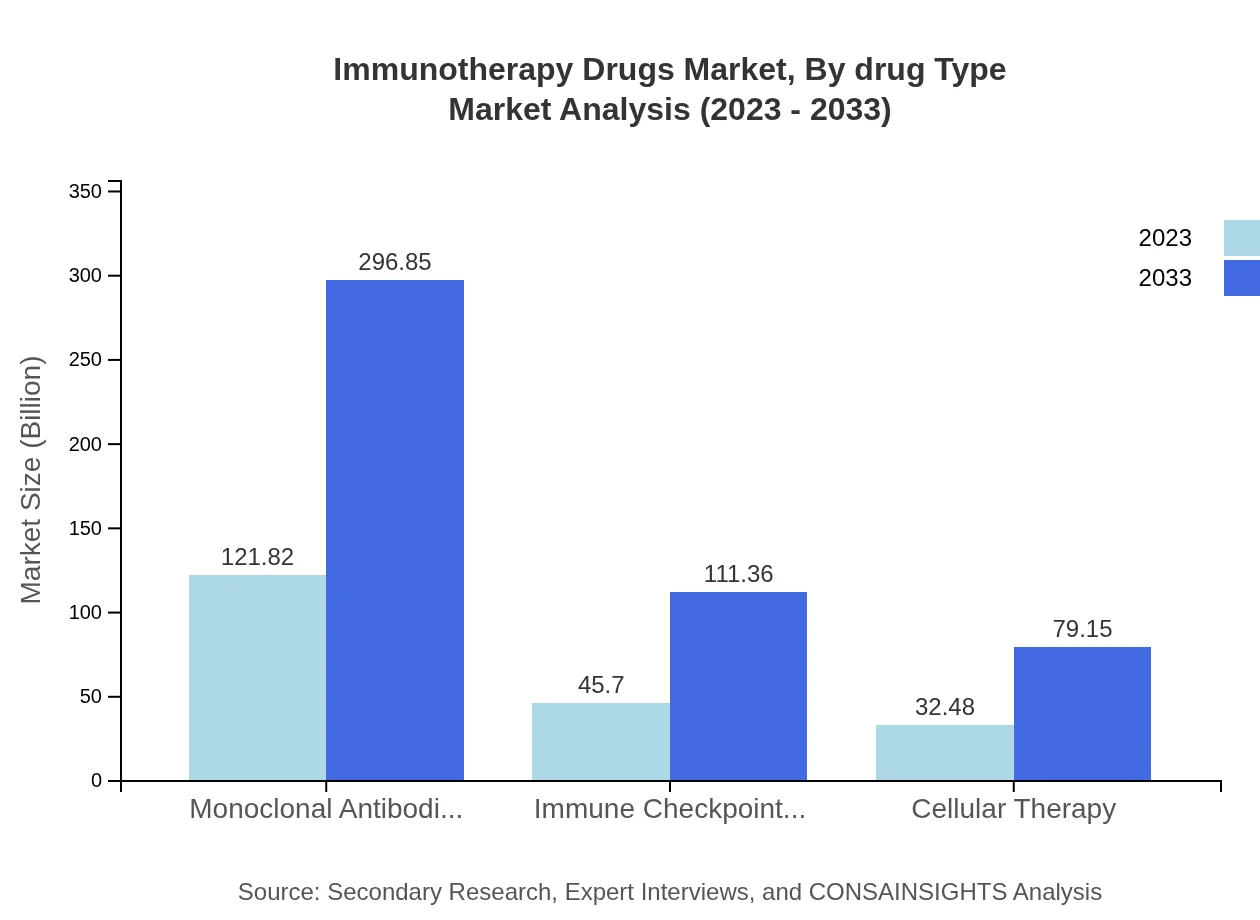
In 2023, the market for monoclonal antibodies was valued at USD 121.82 billion, projected to reach USD 296.85 billion by 2033. Immune checkpoint inhibitors followed with a market size of USD 45.70 billion in 2023, growing to USD 111.36 billion by 2033. Cellular therapy is also gaining traction, with projections from USD 32.48 billion to USD 79.15 billion over the same period, indicating the diversified therapeutic approaches in immunotherapy.
Immunotherapy Drugs Market Analysis By Indication
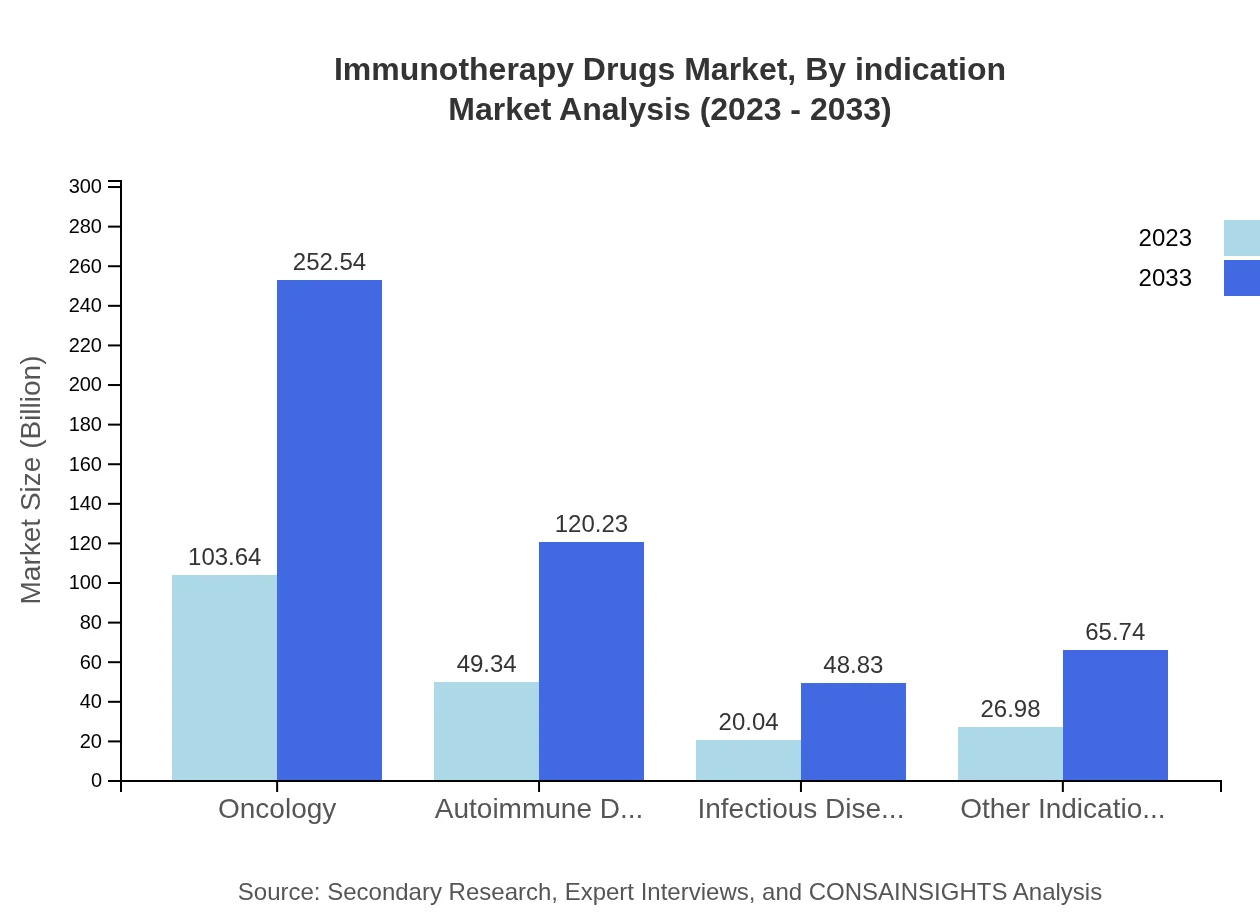
Oncology remains the leading indication for immunotherapy drugs, valued at USD 103.64 billion in 2023, expected to expand to USD 252.54 billion by 2033. Autoimmune disorders segment shows significant growth potential, rising from USD 49.34 billion to USD 120.23 billion. Infectious diseases and other indications are also crucial contributors to the market, reflecting the broad applicability of immunotherapy.
Immunotherapy Drugs Market Analysis By Manufacturer
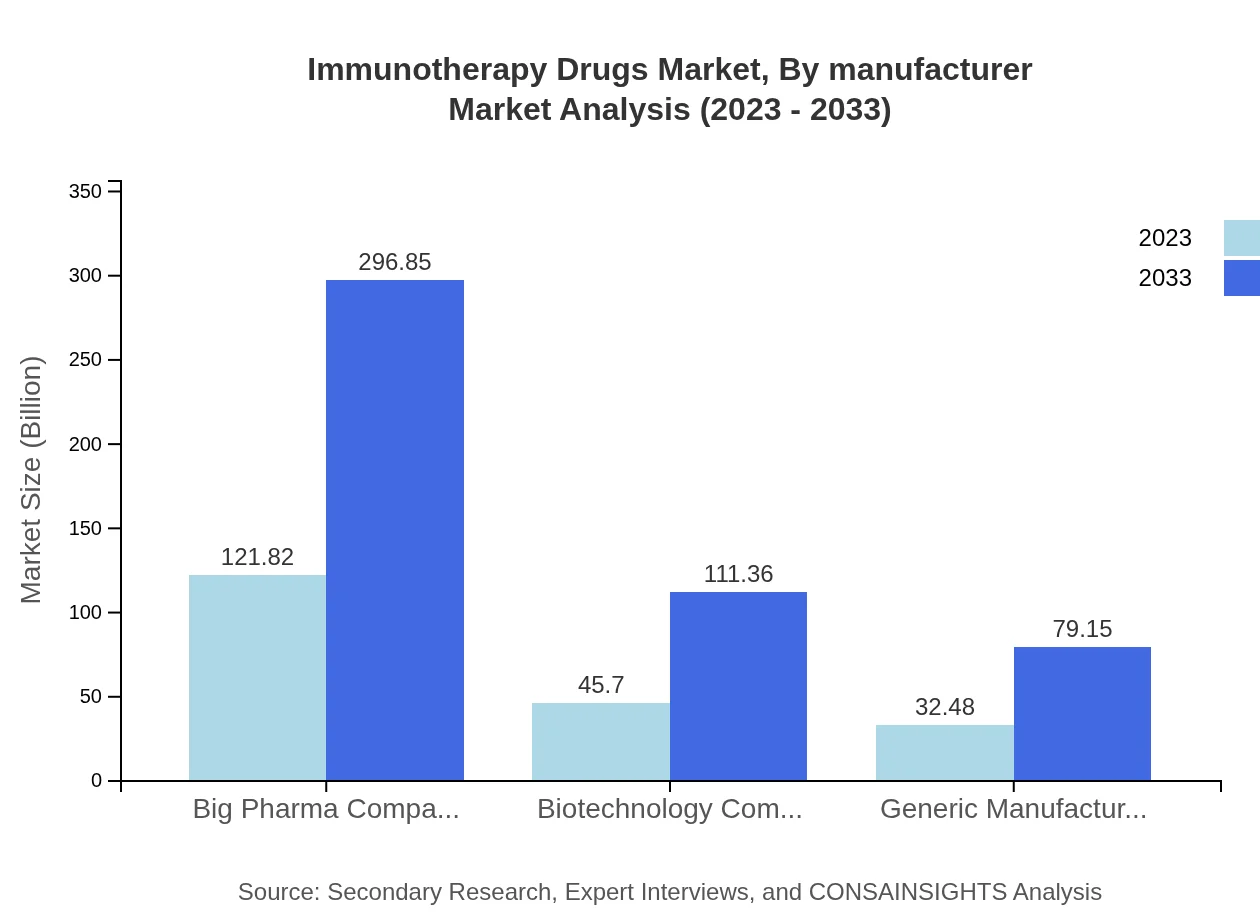
Among manufacturers, Big Pharma companies dominate the market with a share of USD 121.82 billion in 2023, forecasted to reach USD 296.85 billion. Biotechnology companies are also major players, with figures growing from USD 45.70 billion to USD 111.36 billion, demonstrating diverse manufacturing capabilities across the sector.
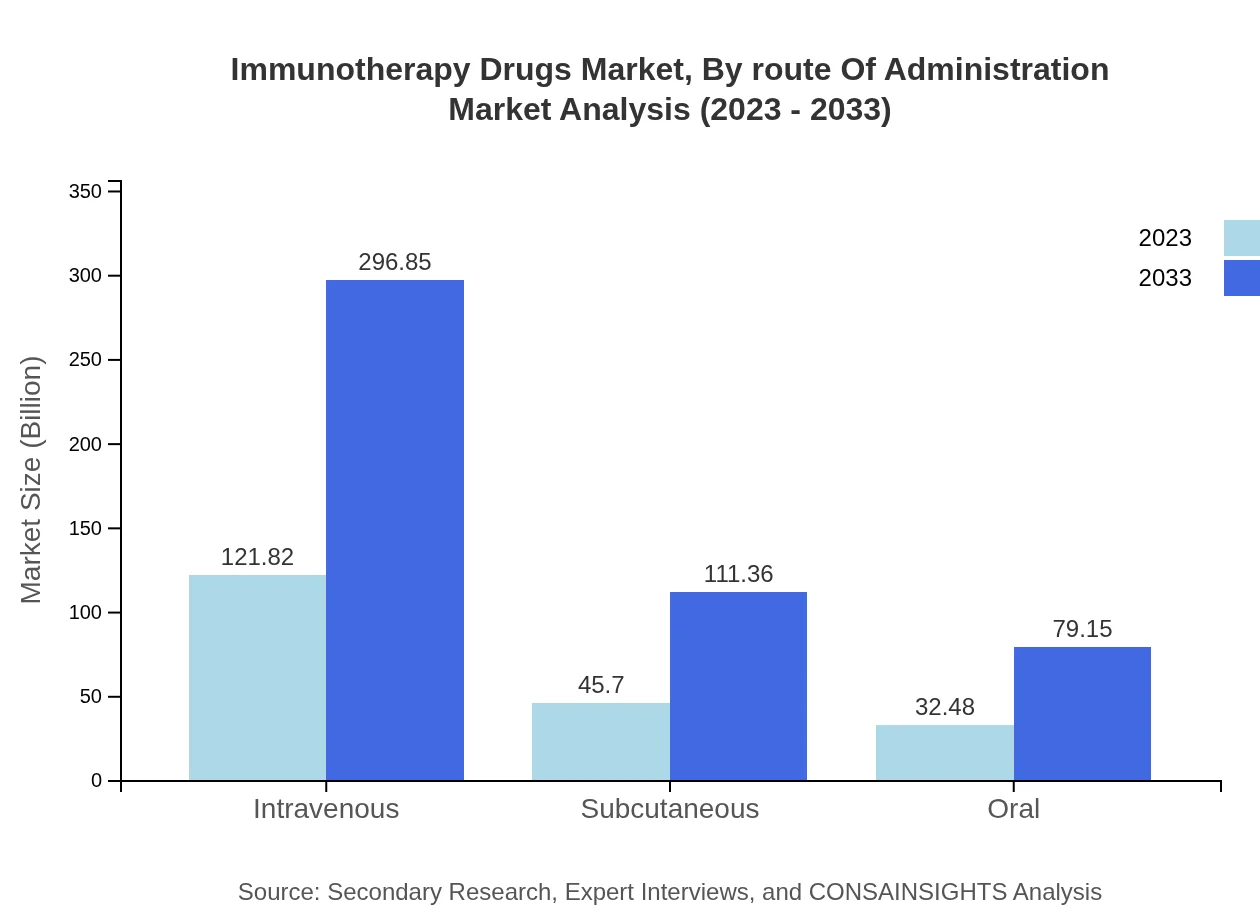
Immunotherapy Drugs Market Analysis By Route Of Administration
The majority of immunotherapy drugs are administered intravenously, valued at USD 121.82 billion in 2023, likely escalating to USD 296.85 billion by 2033. Subcutaneous and oral routes follow, projecting to grow significantly in response to patient preference and convenience, with market sizes moving from USD 45.70 billion and USD 32.48 billion to USD 111.36 billion and USD 79.15 billion respectively.
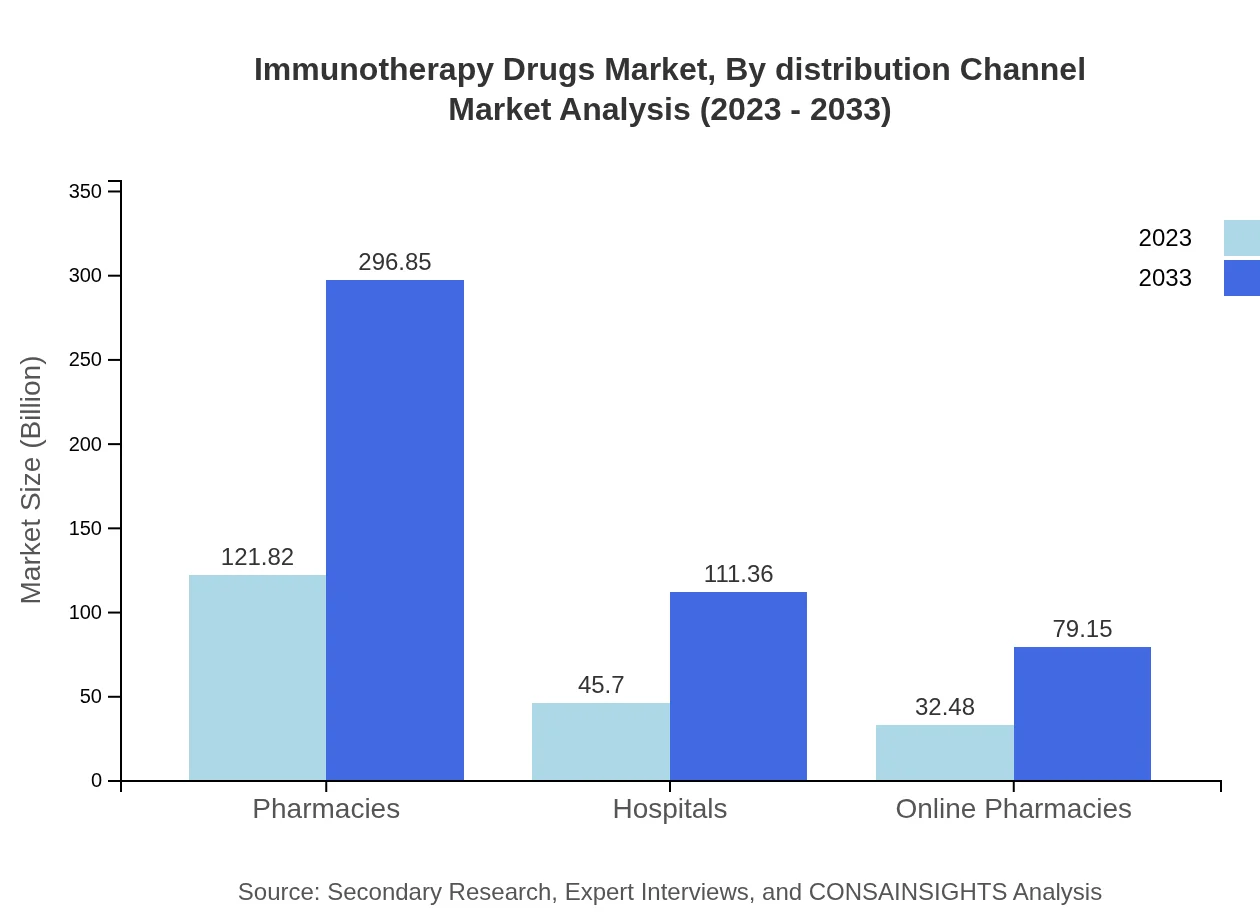
Immunotherapy Drugs Market Analysis By Distribution Channel
Pharmacies lead the distribution channels for immunotherapy drugs, holding a significant share with a market value of USD 121.82 billion in 2023, expanding to USD 296.85 billion. Hospitals represent another critical channel, expected to progress from USD 45.70 billion to USD 111.36 billion, indicating an increasing reliance on hospitals for administering complex therapies.
Immunotherapy Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Immunotherapy Drugs Industry
Roche:
A leader in the development of monoclonal antibodies, Roche has significantly contributed to the immunotherapy landscape with drugs like Rituximab for hematological malignancies.Bristol Myers Squibb:
Recognized for its groundbreaking immune checkpoint inhibitors, Bristol Myers Squibb offers Opdivo and Yervoy that have transformed cancer treatment paradigms.Merck & Co.:
Merck is a pioneer in immunotherapy with its Keytruda product, leading the market in immune-oncology treatments.Pfizer :
Pfizer continues to innovate within the immunotherapy sphere, focusing on both monoclonal antibody and novel therapeutic development.Novartis:
With a strong emphasis on CAR T-cell therapies and gene editing strategies, Novartis is at the forefront of cellular therapy advancements.We're grateful to work with incredible clients.









FAQs
What is the market size of immunotherapy Drugs?
The global immunotherapy drugs market is projected to reach approximately $200 billion by 2033, with a compound annual growth rate (CAGR) of 9%. This growth is driven by increasing cancer cases and advancements in drug development.
What are the key market players or companies in the immunotherapy Drugs industry?
Key players in the immunotherapy drugs industry include major pharmaceutical companies and biotechs focusing on innovative cancer therapies, including monoclonal antibodies, immune checkpoint inhibitors, and cellular therapies.
What are the primary factors driving the growth in the immunotherapy Drugs industry?
Growth in the immunotherapy drugs industry is primarily driven by rising cancer incidences, technological advancements, increased research funding, and improved healthcare infrastructure all contributing to an innovative drug development landscape.
Which region is the fastest Growing in the immunotherapy Drugs?
The Asia Pacific region is the fastest-growing market for immunotherapy drugs, increasing from approximately $36.78 billion in 2023 to $89.62 billion by 2033, reflecting a growing focus on healthcare advancements in this region.
Does ConsaInsights provide customized market report data for the immunotherapy Drugs industry?
Yes, Consainsights offers customized market reports tailored to specific needs in the immunotherapy drugs industry. Clients can receive tailored insights based on detailed market analysis and segmentation.
What deliverables can I expect from this immunotherapy Drugs market research project?
Deliverables from the immunotherapy drugs market research project typically include comprehensive market analysis reports, forecasts, competitive landscape evaluations, and strategic insights tailored to your industry needs.
What are the market trends of immunotherapy Drugs?
Market trends include a significant rise in monoclonal antibodies, with growth from $121.82 billion in 2023 to $296.85 billion by 2033, alongside increasing investments in research and development within the immunotherapy sector.