In Vitro Diagnostics Ivd Quality Control Product Market Report
Published Date: 31 January 2026 | Report Code: in-vitro-diagnostics-ivd-quality-control-product
In Vitro Diagnostics Ivd Quality Control Product Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the In Vitro Diagnostics (IVD) Quality Control Product market, offering insights on market size, trends, regional dynamics, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
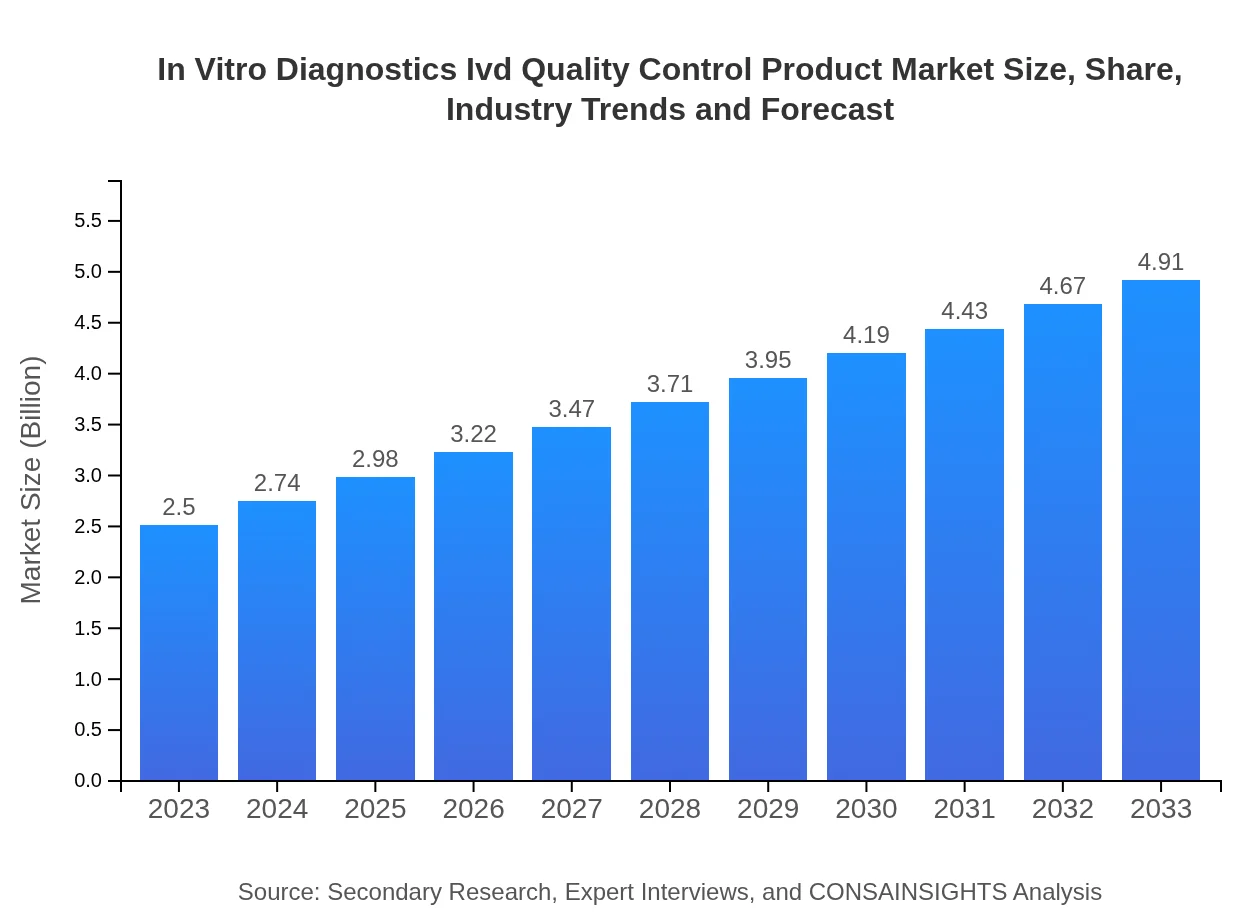
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
In Vitro Diagnostics Ivd Quality Control Product Market Overview
Customize In Vitro Diagnostics Ivd Quality Control Product Market Report market research report
- ✔ Get in-depth analysis of In Vitro Diagnostics Ivd Quality Control Product market size, growth, and forecasts.
- ✔ Understand In Vitro Diagnostics Ivd Quality Control Product's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in In Vitro Diagnostics Ivd Quality Control Product
What is the Market Size & CAGR of In Vitro Diagnostics Ivd Quality Control Product market in {Year}?
In Vitro Diagnostics Ivd Quality Control Product Industry Analysis
In Vitro Diagnostics Ivd Quality Control Product Market Segmentation and Scope
Tell us your focus area and get a customized research report.
In Vitro Diagnostics Ivd Quality Control Product Market Analysis Report by Region
Europe In Vitro Diagnostics Ivd Quality Control Product Market Report:
European markets are projected to expand from $0.70 billion in 2023 to $1.38 billion in 2033, supported by stringent regulatory frameworks and a focus on enhancing diagnostic testing accuracy.Asia Pacific In Vitro Diagnostics Ivd Quality Control Product Market Report:
In Asia Pacific, the IVD Quality Control Product market is projected to grow from $0.54 billion in 2023 to $1.07 billion by 2033. The rising healthcare infrastructure, increasing prevalence of diseases, and growing investments in medical technology drive this growth.North America In Vitro Diagnostics Ivd Quality Control Product Market Report:
North America remains the largest market, expected to grow from $0.88 billion in 2023 to $1.73 billion by 2033, fueled by high healthcare spending, advanced healthcare infrastructure, and increasing diagnostic procedures.South America In Vitro Diagnostics Ivd Quality Control Product Market Report:
The South America region shows a slower growth trajectory, with the market increasing from $0.07 billion in 2023 to $0.14 billion in 2033. Factors such as economic challenges and limited access to advanced healthcare technologies contribute to this slow growth rate.Middle East & Africa In Vitro Diagnostics Ivd Quality Control Product Market Report:
In the Middle East and Africa, the market is anticipated to grow from $0.30 billion in 2023 to $0.60 billion by 2033, driven by improvements in healthcare provision and increasing awareness about the importance of quality control in diagnostics.Tell us your focus area and get a customized research report.
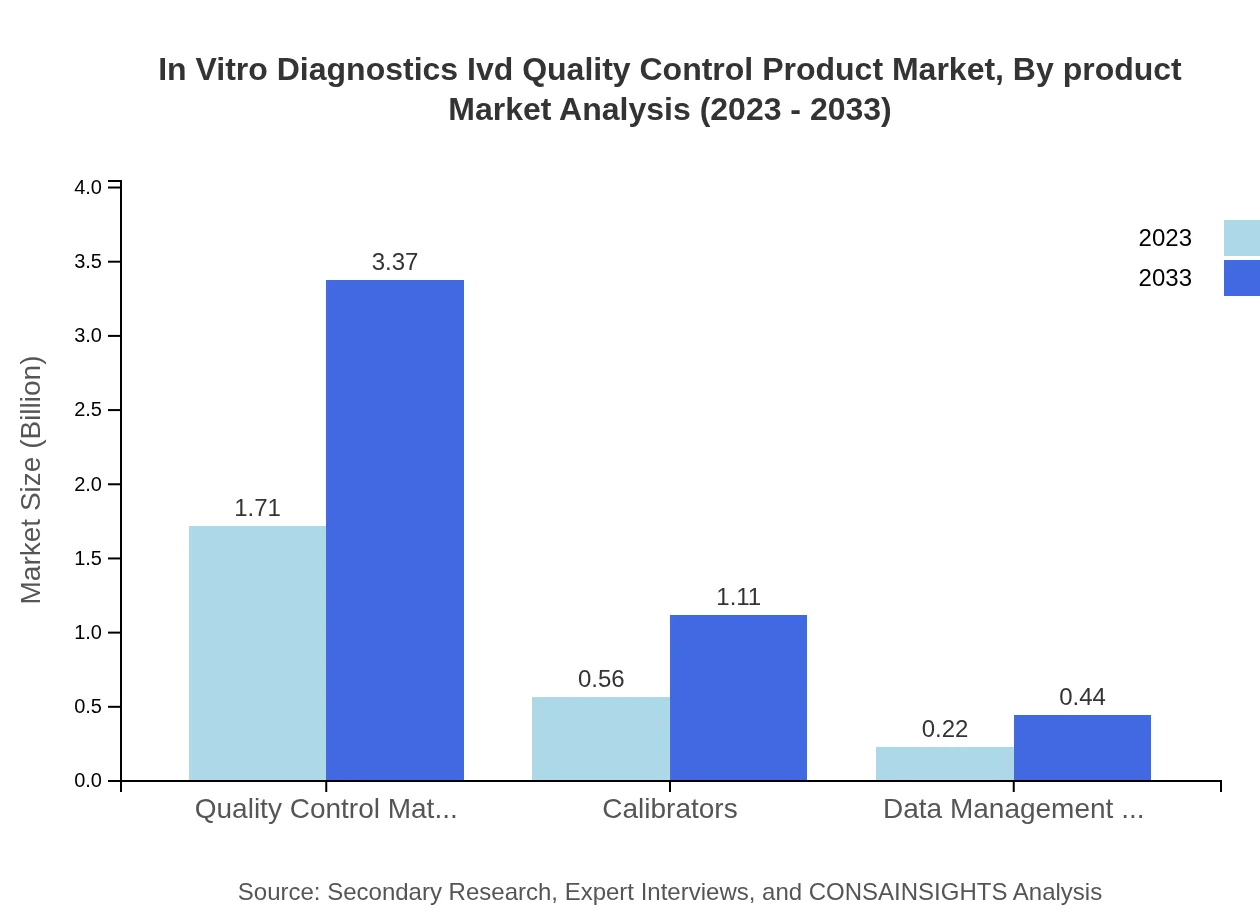
In Vitro Diagnostics Ivd Quality Control Product Market Analysis By Product
The quality control materials segment, valued at $1.71 billion in 2023, is projected to reach $3.37 billion by 2033, maintaining its leadership in the market with a consistent share of approximately 68.55%. Other segments such as calibrators and data management software are also experiencing growth, indicating a shifting landscape within quality control methodologies.
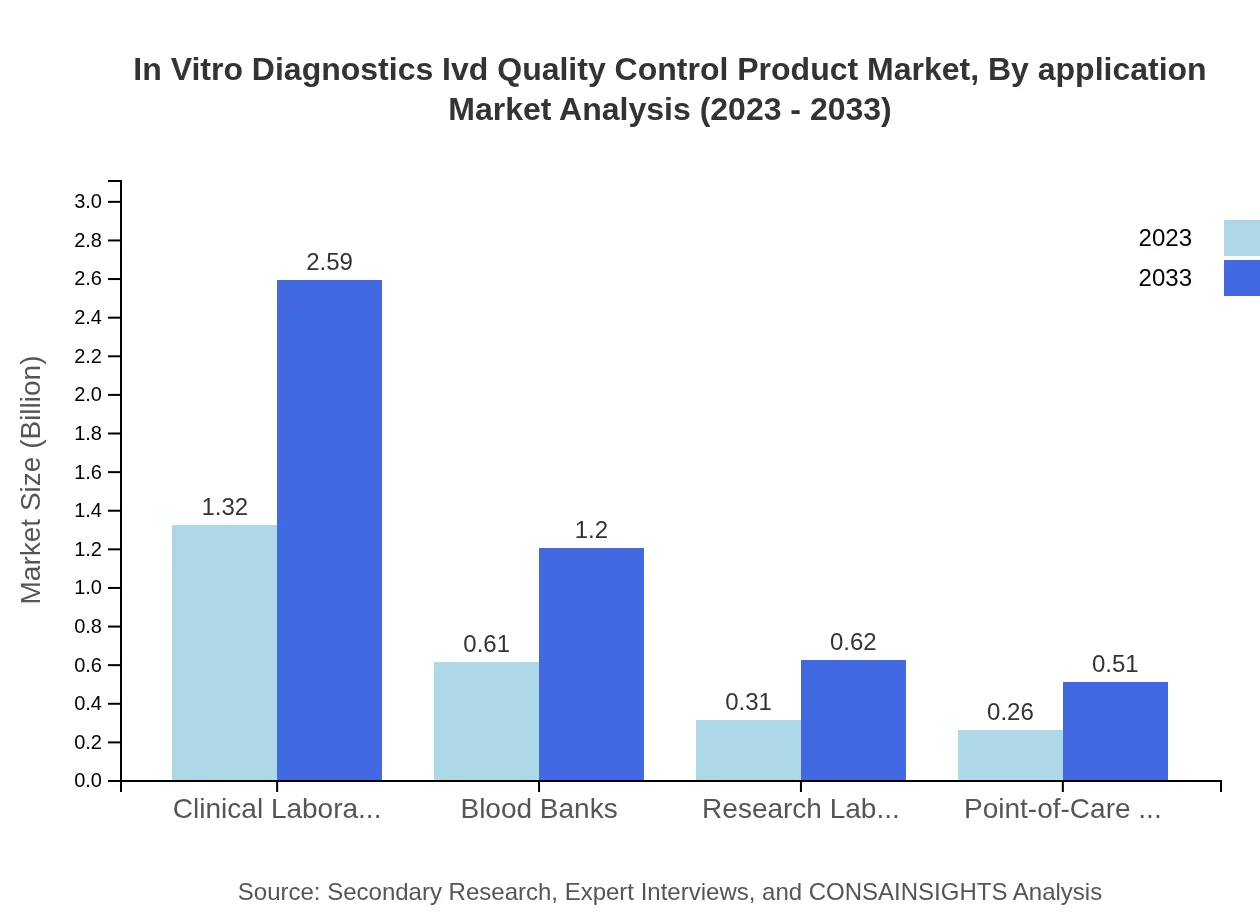
In Vitro Diagnostics Ivd Quality Control Product Market Analysis By Application
By application, the Clinical Laboratories segment is predominant, expected to hold around 52.77% of the market share through to 2033. This focus on clinical applications highlights the emphasis on accurate diagnostics in patient care.
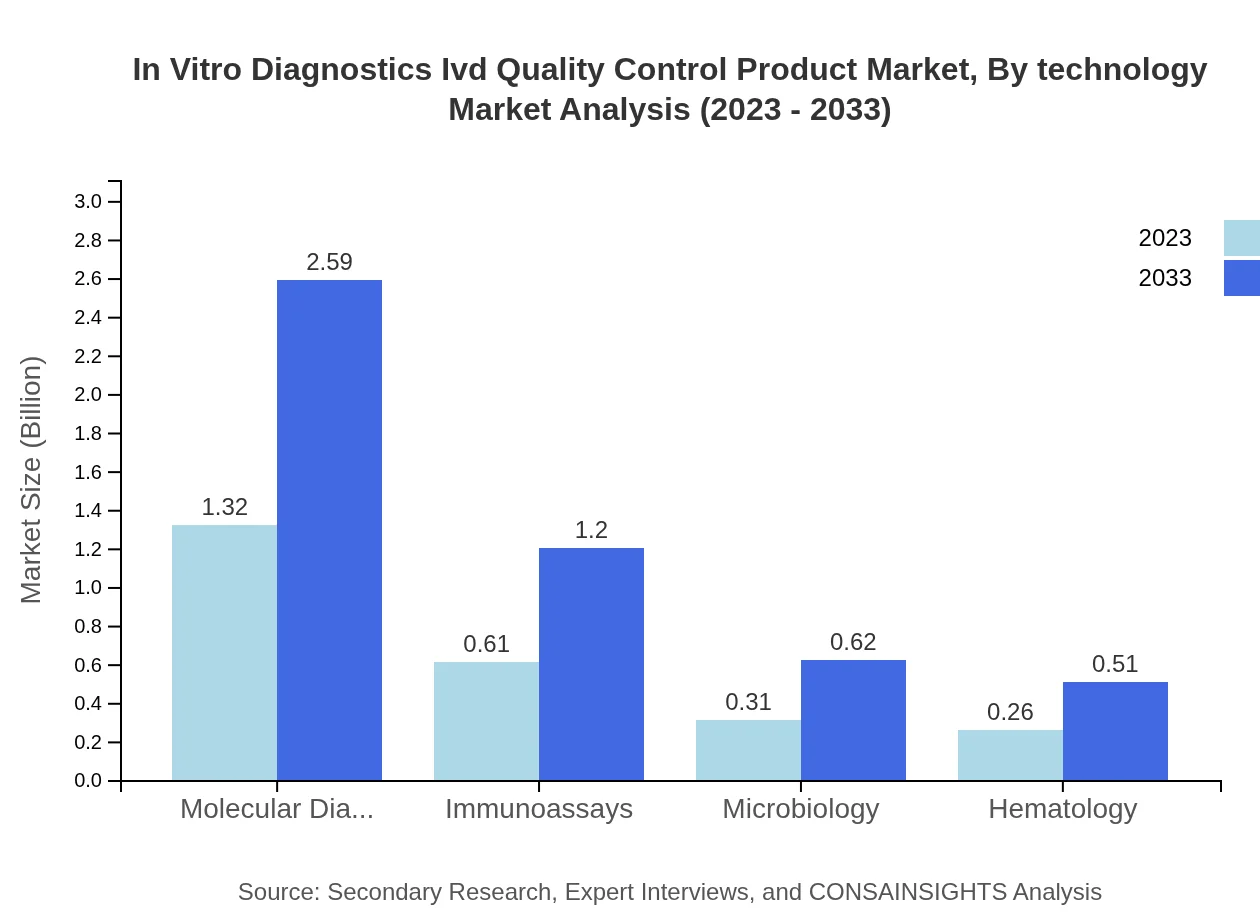
In Vitro Diagnostics Ivd Quality Control Product Market Analysis By Technology
Emerging technologies are reshaping the IVD sector, with automation and software solutions gaining traction. This transition is leading to enhanced data management and accuracy in quality control efforts across diagnostic processes.
In Vitro Diagnostics Ivd Quality Control Product Market Analysis By Product_type
Global In-Vitro Diagnostics (IVD) Quality Control Product Market, By Product Type Market Analysis (2023 - 2033)
Significant advancements in quality control products are influenced by their types, with manual control products maintaining a dominant share of 87.39%. This segment's robust performance is indicative of the industry's reliance on manual interventions in achieving accurate diagnostics.
In Vitro Diagnostics Ivd Quality Control Product Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in In Vitro Diagnostics Ivd Quality Control Product Industry
Abbott Laboratories:
Abbott is a global healthcare leader that engages in the research, development, and manufacture of healthcare products, focusing significantly on diagnostics and innovative quality control solutions.Roche Diagnostics:
Roche is a pioneer in the diagnostics industry, offering a wide array of quality control products aimed at enhancing laboratory performance and improving patient outcomes.Siemens Healthineers:
Siemens Healthineers specializes in medical technology, providing cutting-edge IVD solutions, including comprehensive quality control products that ensure the accuracy and efficiency of diagnostic tests.Thermo Fisher Scientific:
A leader in providing analytical instruments, Thermo Fisher focuses on comprehensive solutions in the IVD segment, including innovative quality control measures that cater to various diagnostic needs.We're grateful to work with incredible clients.









FAQs
What is the market size of in Vitro Diagnostics Ivd Quality Control Product?
The global market size for In-Vitro Diagnostics (IVD) Quality Control Products is estimated at $2.5 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.8% through to 2033.
What are the key market players or companies in this in Vitro Diagnostics Ivd Quality Control Product industry?
Key players in the IVD quality control market include established companies such as Roche Diagnostics, Sysmex Corporation, Siemens Healthineers, and bioMérieux. These companies drive innovation, enhance product offerings and are pivotal in market expansions.
What are the primary factors driving the growth in the in Vitro Diagnostics Ivd Quality Control Product industry?
The growth drivers of the IVD quality control market include the rising prevalence of chronic diseases, advancements in diagnostic technologies, an increasing focus on healthcare quality, and strong regulatory frameworks boosting product demand across global markets.
Which region is the fastest Growing in the in Vitro Diagnostics Ivd Quality Control Product?
The Asia Pacific region is projected as the fastest-growing market for IVD quality control products, expanding from $0.54 billion in 2023 to $1.07 billion by 2033, fueled by rising healthcare investments and enhanced diagnostic capabilities.
Does ConsaInsights provide customized market report data for the in Vitro Diagnostics Ivd Quality Control Product industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the IVD quality control segment, ensuring that our clients receive insights aligned with their strategic objectives and market dynamics.
What deliverables can I expect from this in Vitro Diagnostics Ivd Quality Control Product market research project?
Deliverables from the IVD quality control market research project typically include comprehensive reports with market size, trends, segment analysis, forecasts, competitive landscape, and actionable insights to aid in strategic planning.
What are the market trends of in Vitro Diagnostics Ivd Quality Control Product?
Current trends in the IVD quality control products market show increased demand for molecular diagnostics, automation in labs, a shift toward manual control products, and growth in software for data management, indicating a trend towards improved efficiency and accuracy.