In Vivo Toxicology Market Report
Published Date: 31 January 2026 | Report Code: in-vivo-toxicology
In Vivo Toxicology Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the In Vivo Toxicology market, detailing market trends, size, segmentation, and projections from 2023 to 2033, alongside key insights into regional developments and competitive landscapes.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
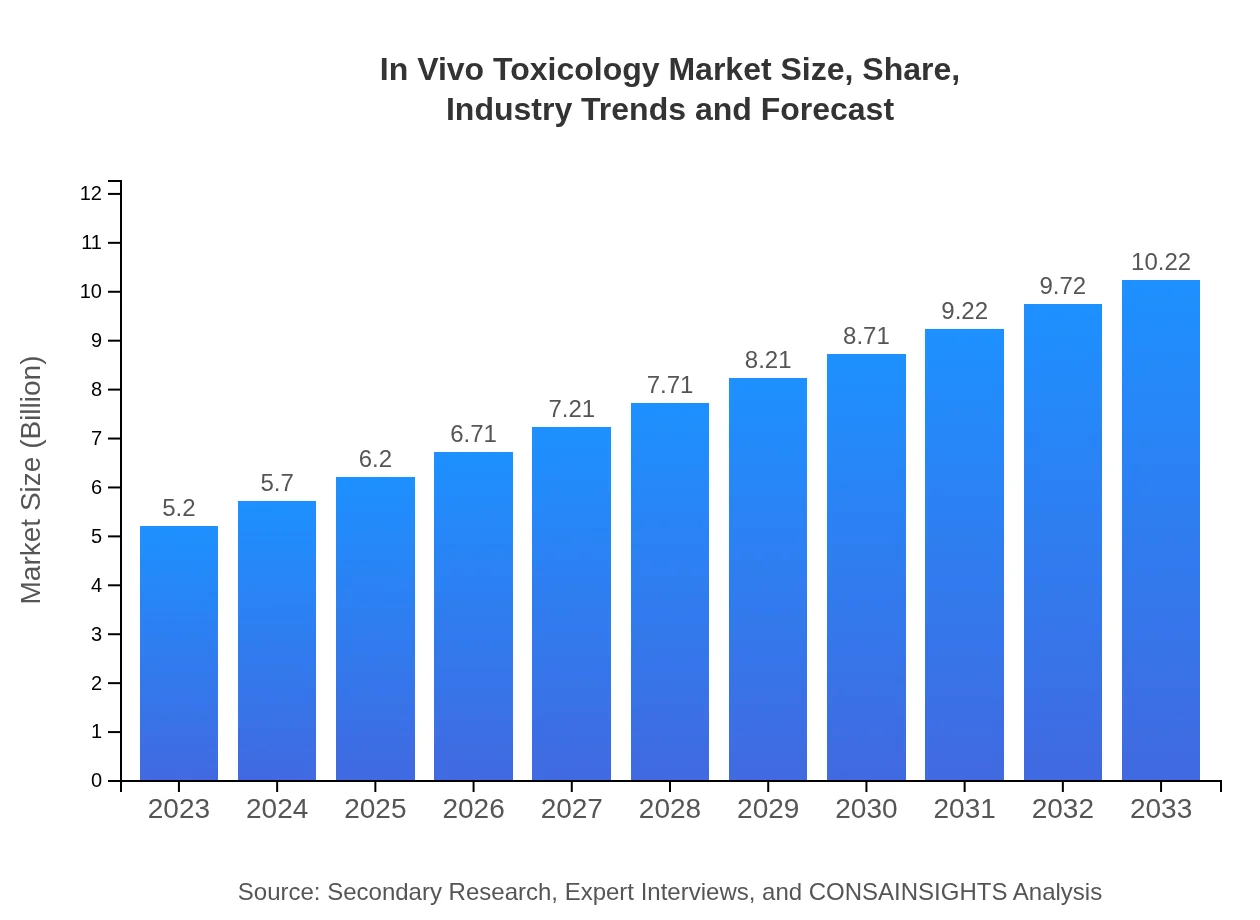
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Charles River Laboratories, Covance, WuXi AppTec, Eurofins Scientific, Invivo Therapeutics |
| Last Modified Date | 31 January 2026 |
In Vivo Toxicology Market Overview
Customize In Vivo Toxicology Market Report market research report
- ✔ Get in-depth analysis of In Vivo Toxicology market size, growth, and forecasts.
- ✔ Understand In Vivo Toxicology's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in In Vivo Toxicology
What is the Market Size & CAGR of In Vivo Toxicology market in 2023?
In Vivo Toxicology Industry Analysis
In Vivo Toxicology Market Segmentation and Scope
Tell us your focus area and get a customized research report.
In Vivo Toxicology Market Analysis Report by Region
Europe In Vivo Toxicology Market Report:
Europe's market is anticipated to grow from $1.31 billion in 2023 to $2.58 billion by 2033. The region benefits from advanced research infrastructure and advocacy for the reduction of animal testing, contributing to the growth of alternative testing methods.Asia Pacific In Vivo Toxicology Market Report:
The Asia Pacific region is anticipated to experience substantial growth, with the market size expected to rise from $1.10 billion in 2023 to $2.16 billion by 2033. This growth is attributed to increasing investments in biotechnology and pharmaceuticals, along with a growing prevalence of chronic diseases necessitating innovative treatment options.North America In Vivo Toxicology Market Report:
North America leads the In Vivo Toxicology market with an estimated size of $1.96 billion in 2023, projected to grow to $3.85 billion by 2033. Strong research funding, presence of major market players, and stringent regulations are vital factors propelling this market.South America In Vivo Toxicology Market Report:
In South America, the In Vivo Toxicology market is projected to grow from $0.40 billion in 2023 to $0.78 billion by 2033. The rise is driven by expanding research activities and growing demand for toxicological evaluations within pharmaceutical and agricultural sectors.Middle East & Africa In Vivo Toxicology Market Report:
The Middle East and Africa are expected to witness gradual growth, with market size anticipated to increase from $0.43 billion in 2023 to $0.85 billion by 2033. The focus on improving healthcare and pharmaceutical sectors is expected to drive demand for toxicology assessments.Tell us your focus area and get a customized research report.
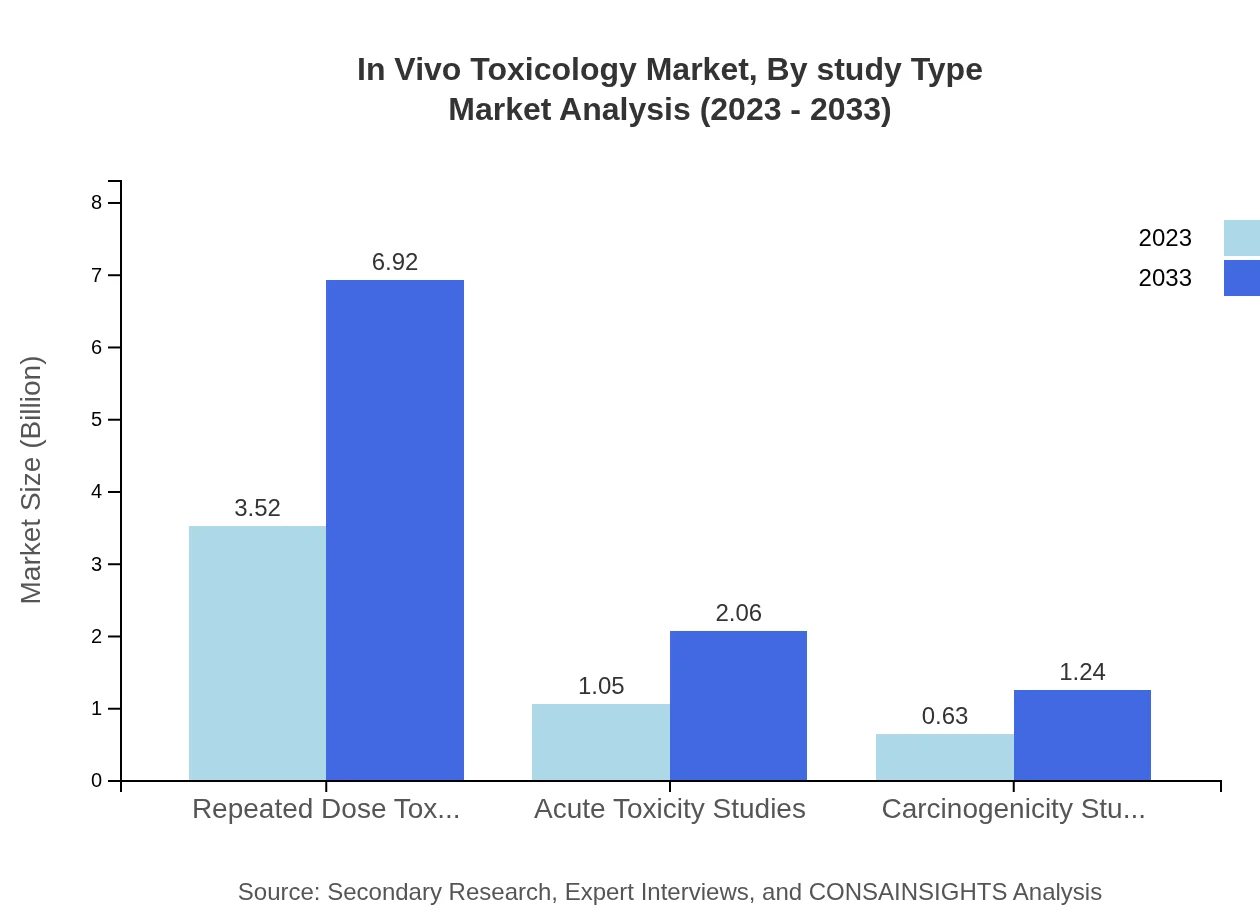
In Vivo Toxicology Market Analysis By Study Type
Study types such as repeated dose toxicity studies dominate the market, increasing from $3.52 billion in 2023 to $6.92 billion by 2033. Observational studies follow closely, also showing promising growth. The focus on safety and efficacy necessitates comprehensive assessments across study types.
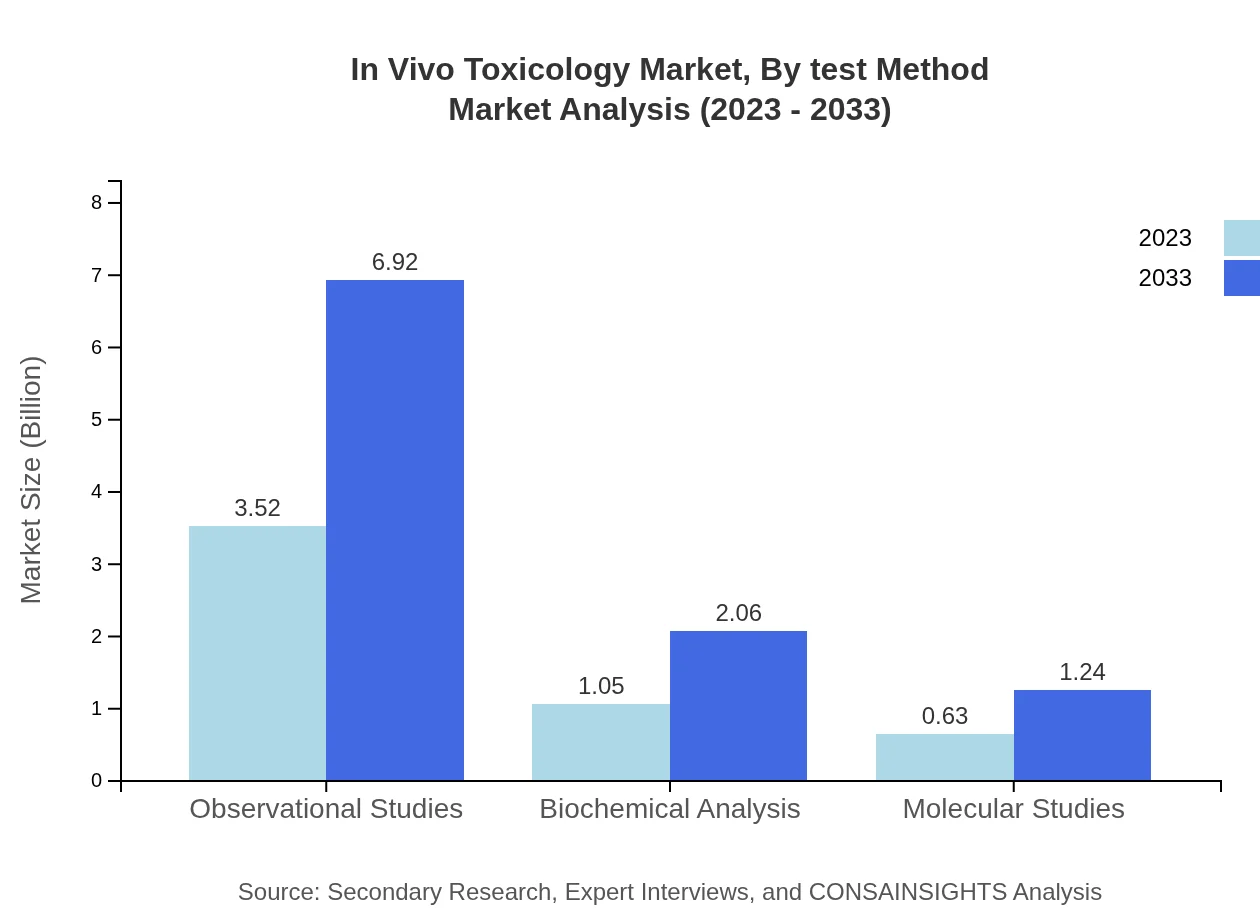
In Vivo Toxicology Market Analysis By Test Method
The market shows diverse test methods, with acute toxicity studies growing from $1.05 billion in 2023 to $2.06 billion by 2033. The demand for diverse methods stems from regulatory requirements and the need for detailed toxicological profiles.
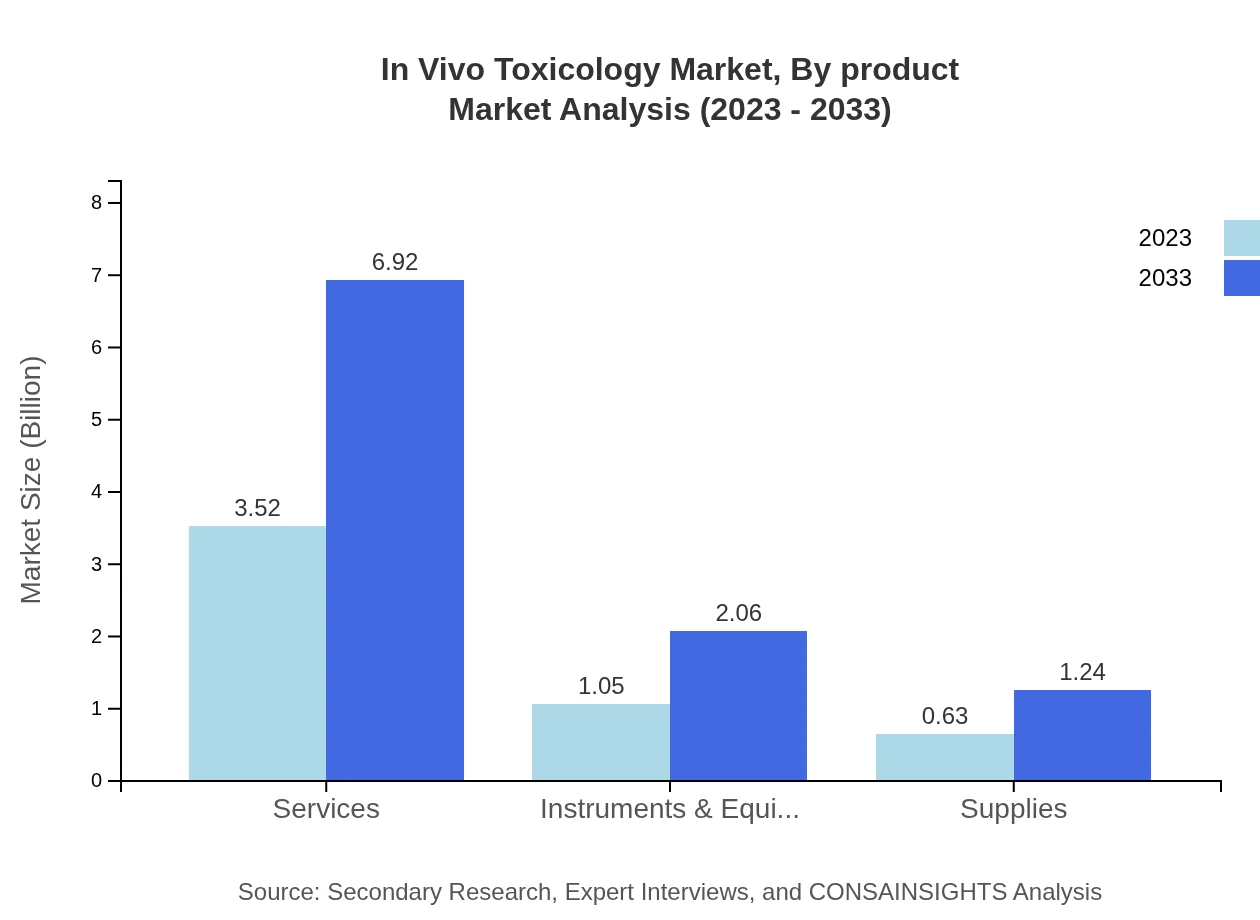
In Vivo Toxicology Market Analysis By Product
The product segmentation includes services, instruments, and supplies. Services dominate the market, projected to expand significantly due to the increase in drug testing programs and custom toxicology services across industries.
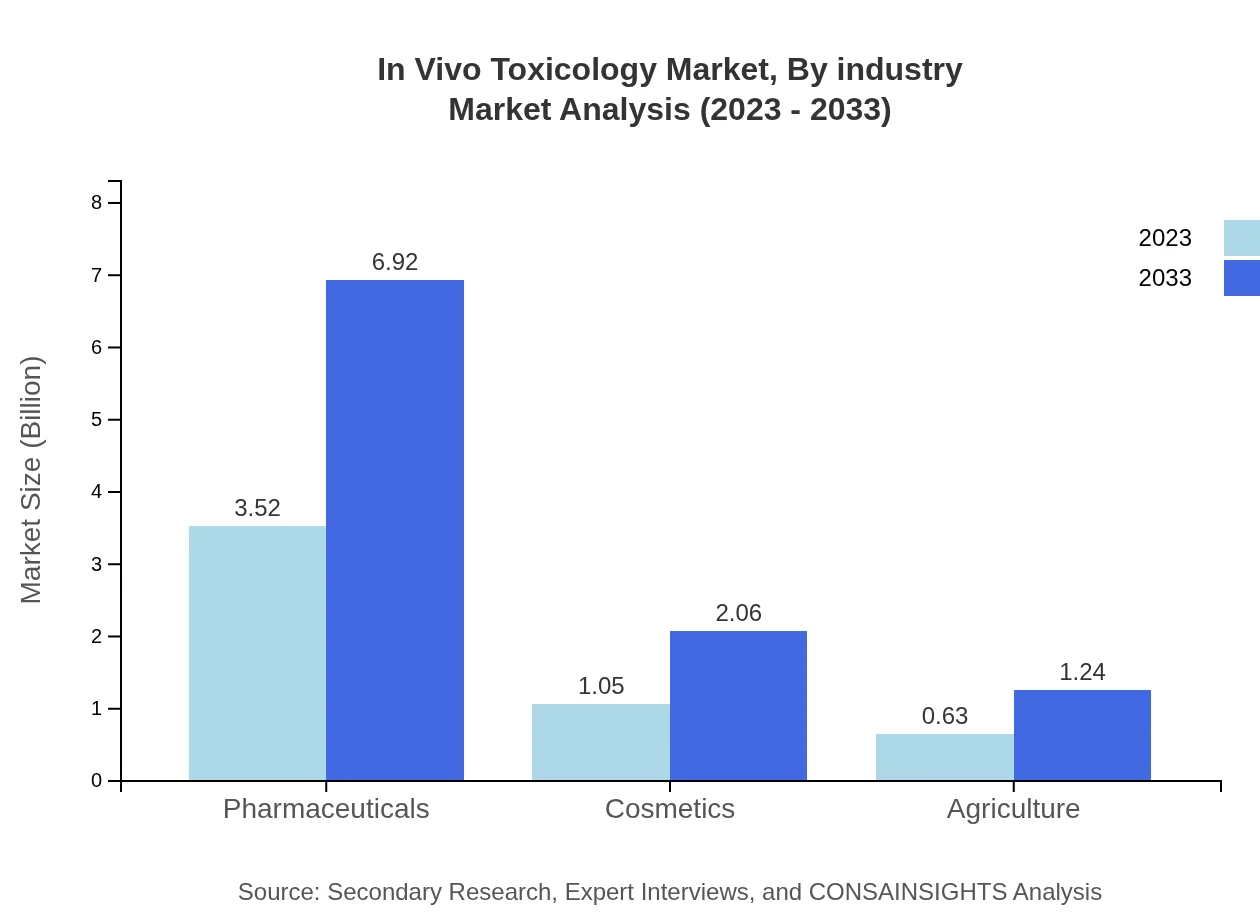
In Vivo Toxicology Market Analysis By Industry
The pharmaceutical industry remains the largest segment, with continued investment in research and development. Growth in the cosmetics and agricultural industries is also notable, driven by regulatory pushes for safer products.
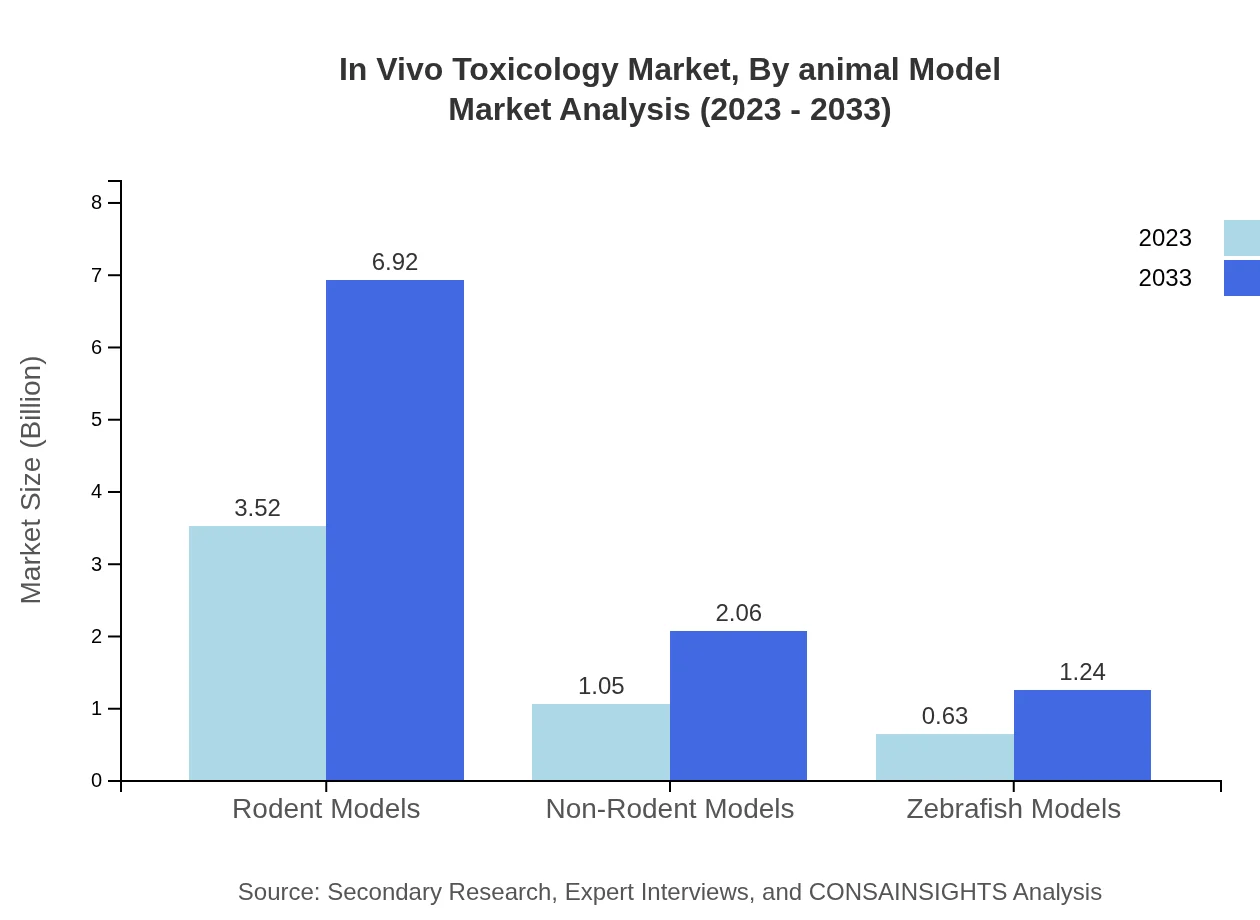
In Vivo Toxicology Market Analysis By Animal Model
Rodent models are prominently used in toxicity testing, accounting for significant market share. However, the adoption of non-rodent and zebrafish models is rising due to evolving study requirements and ethical considerations.
In Vivo Toxicology Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in In Vivo Toxicology Industry
Charles River Laboratories:
A leading provider of laboratory services known for drug development and safety assessment, offering comprehensive in vivo toxicology solutions.Covance:
Part of Labcorp, Covance is a major player in drug development services specializing in in vivo toxicology and providing specialized testing across various sectors.WuXi AppTec:
Offers a broad range of services including in vivo toxicology studies, focusing on accelerating drug development through innovative assessment methodologies.Eurofins Scientific:
Provides independent product testing and support services, specializing in toxicology, and contributing to regulatory compliance across multiple industries.Invivo Therapeutics:
Focused on developing advanced technologies in therapeutic efficacy and safety assessments through improved in vivo models.We're grateful to work with incredible clients.









FAQs
What is the market size of in Vivo Toxicology?
The global in-vivo toxicology market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 6.8% from 2023 to 2033. This growth is driven by increasing regulatory requirements for drug testing.
What are the key market players or companies in this in Vivo Toxicology industry?
Key players in the in-vivo toxicology industry include Charles River Laboratories, Covance, Envigo, and WuXi AppTec. These companies are prominent for their comprehensive toxicology services and innovative testing methodologies.
What are the primary factors driving the growth in the in Vivo Toxicology industry?
Growth in the in-vivo toxicology sector is primarily driven by increasing investments in drug discovery, stringent regulatory frameworks, and the rising need for safety assessments in pharmaceuticals, agriculture, and cosmetics.
Which region is the fastest Growing in the in Vivo Toxicology?
Asia-Pacific is the fastest-growing region in the in-vivo toxicology market, expanding from $1.10 billion in 2023 to $2.16 billion by 2033. This growth is attributed to increased research activities and affordable outsourcing options.
Does ConsaInsights provide customized market report data for the in Vivo Toxicology industry?
Yes, ConsaInsights offers customized market report data tailored to specific requirements in the in-vivo toxicology industry, allowing businesses to acquire insights relevant to their market strategies.
What deliverables can I expect from this in Vivo Toxicology market research project?
Deliverables include comprehensive market analysis reports, competitor analysis, growth forecasts, and segmentation insights, enabling clients to make informed business decisions in the in-vivo toxicology arena.
What are the market trends of in Vivo Toxicology?
Current trends in the in-vivo toxicology market highlight increasing automation in testing processes, the adoption of alternative testing methods, and a growing shift towards personalized medicine, enhancing the efficiency of drug development.