Infection Prevention Enteral Access Market Report
Published Date: 31 January 2026 | Report Code: infection-prevention-enteral-access
Infection Prevention Enteral Access Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Infection Prevention Enteral Access market, providing insights such as market size, growth trends, regional variances, industry analyses, and forecasts for the period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
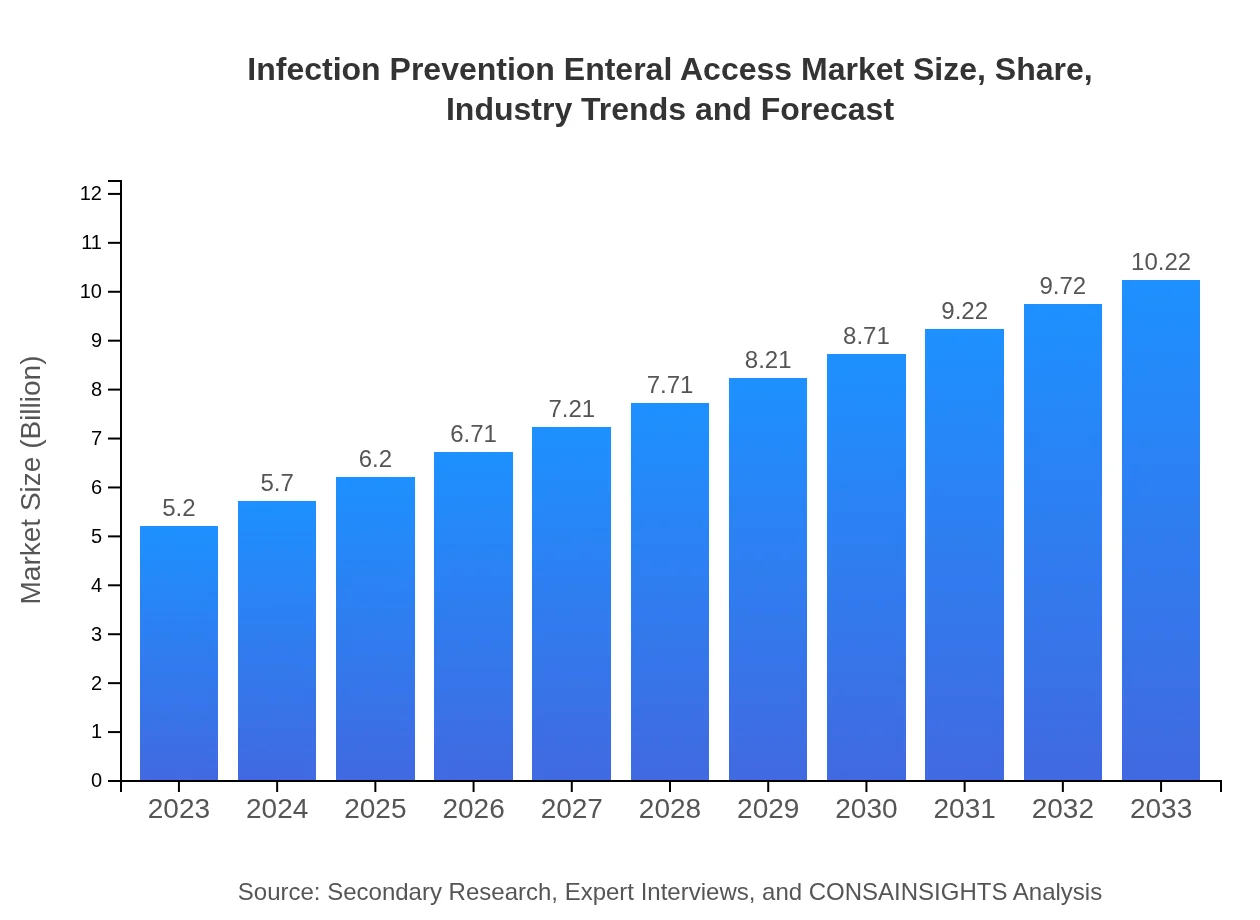
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Abbott Laboratories, Medtronic , Boston Scientific, Cook Medical, B. Braun Melsungen AG |
| Last Modified Date | 31 January 2026 |
Infection Prevention Enteral Access Market Overview
Customize Infection Prevention Enteral Access Market Report market research report
- ✔ Get in-depth analysis of Infection Prevention Enteral Access market size, growth, and forecasts.
- ✔ Understand Infection Prevention Enteral Access's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Infection Prevention Enteral Access
What is the Market Size & CAGR of the Infection Prevention Enteral Access market in 2023?
Infection Prevention Enteral Access Industry Analysis
Infection Prevention Enteral Access Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Infection Prevention Enteral Access Market Analysis Report by Region
Europe Infection Prevention Enteral Access Market Report:
Europe's market is assessed at $1.51 billion in 2023, with projections of $2.96 billion by 2033. The region's focus on enhancing healthcare quality and stringent regulatory measures for infection prevention are key growth drivers.Asia Pacific Infection Prevention Enteral Access Market Report:
In the Asia Pacific region, the market size is valued at $0.98 billion in 2023 and is expected to grow to $1.93 billion by 2033. Factors contributing to this growth include rising healthcare investments, a growing aging population, and increasing awareness of infection prevention practices.North America Infection Prevention Enteral Access Market Report:
North America remains the leading region with a market size of $1.99 billion in 2023, forecasted to reach $3.91 billion by 2033. Advanced healthcare infrastructure, a high prevalence of chronic diseases, and strong governmental support for infection control propel growth.South America Infection Prevention Enteral Access Market Report:
South America has a market size of $0.30 billion in 2023, projected to increase to $0.59 billion by 2033. Increasing healthcare access and patient safety regulations will enhance market demand in this region as enteral feeding solutions become more common.Middle East & Africa Infection Prevention Enteral Access Market Report:
The Middle East and Africa exhibit a growing market, standing at $0.42 billion in 2023 and expected to reach $0.83 billion by 2033. Improving healthcare services and rising awareness regarding infection prevention methods contribute to this growth.Tell us your focus area and get a customized research report.
Infection Prevention Enteral Access Market Analysis By Product
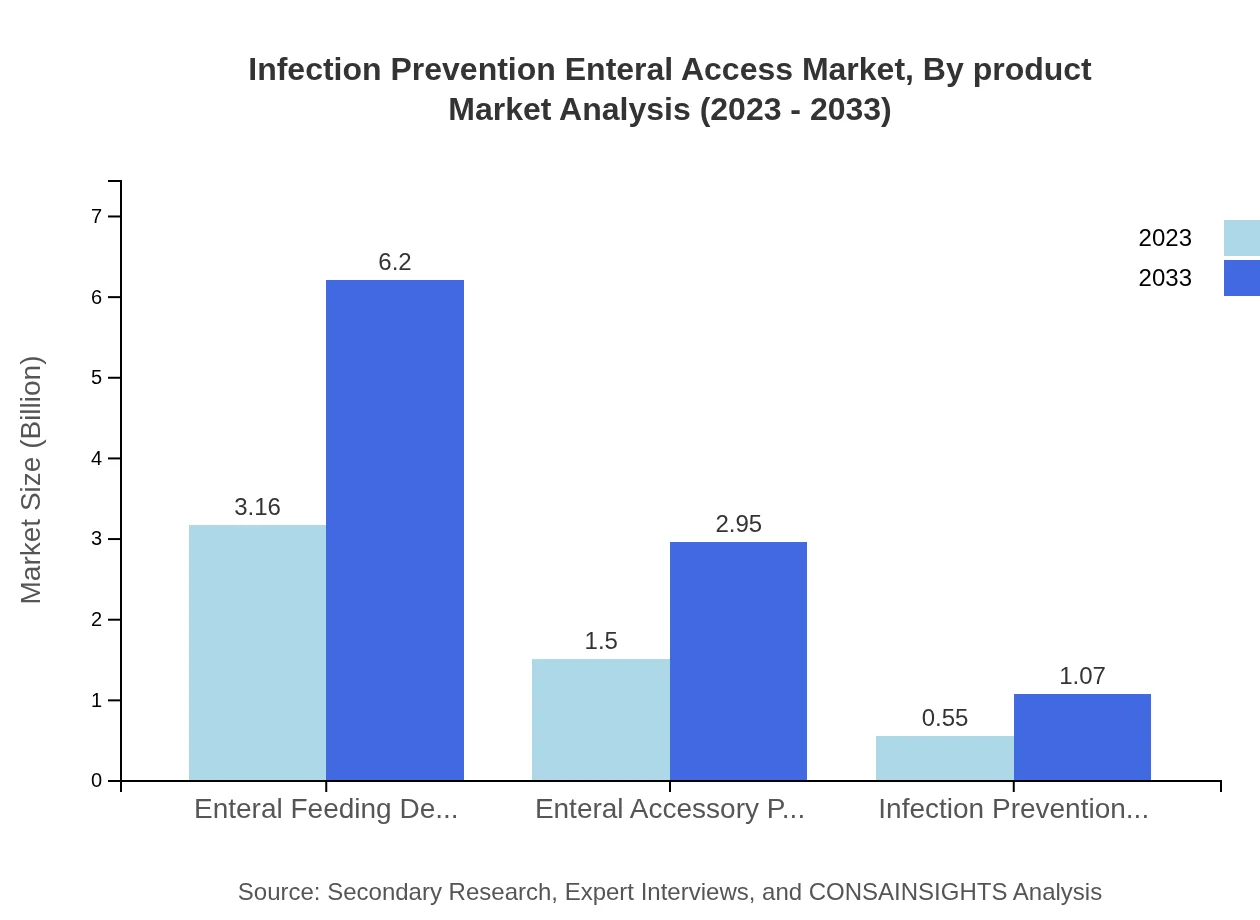
The Infection Prevention Enteral Access market is primarily driven by product types, including Enteral Feeding Devices, which dominate with a market share of 60.68% in 2023, valued at $3.16 billion. Following this are Enteral Accessory Products with a 28.82% share or $1.50 billion, and Infection Prevention Devices at 10.5% or $0.55 billion, reflecting their essential roles in enhancing patient care.
Infection Prevention Enteral Access Market Analysis By Procedure
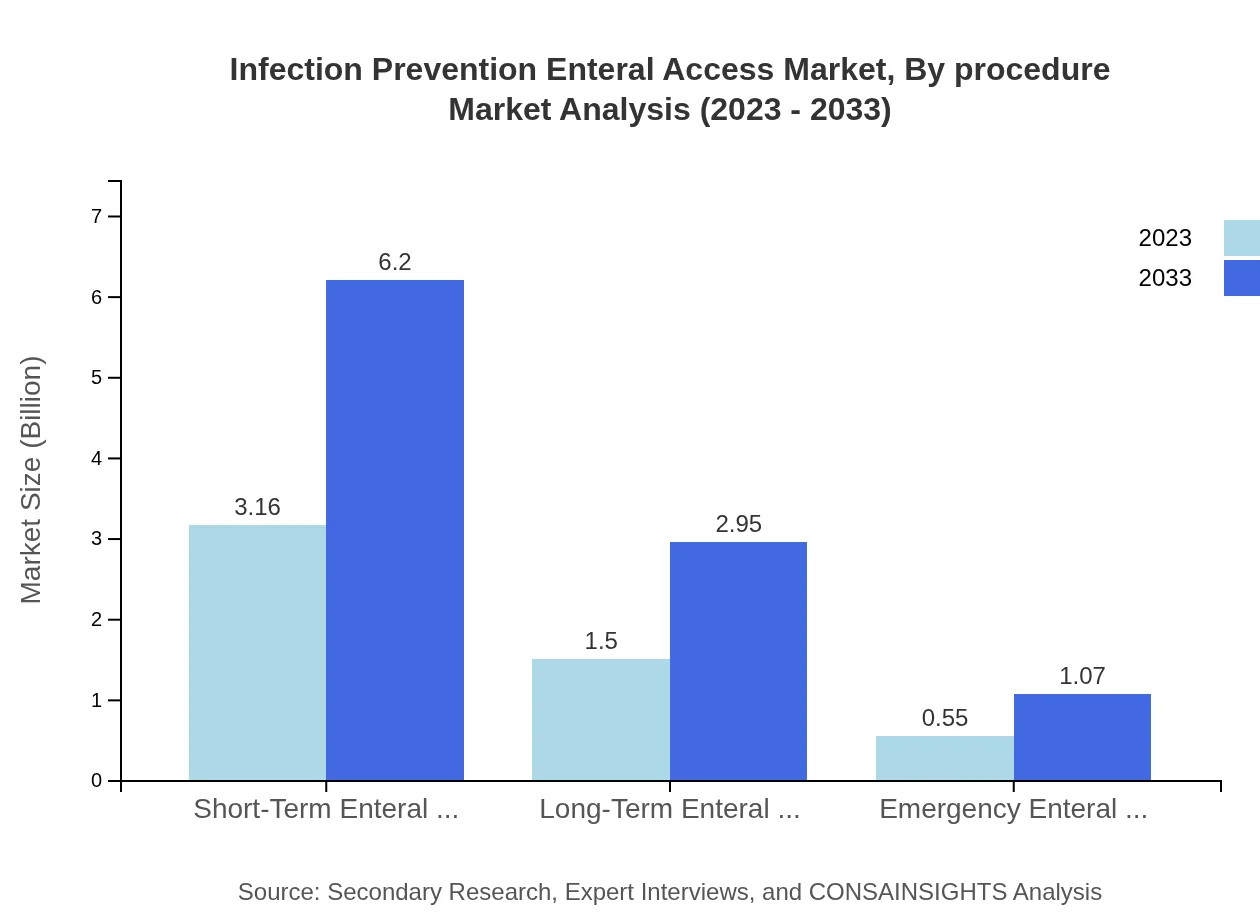
Analysis by procedure reveals that Short-Term Enteral Access holds the majority market share of 60.68% in 2023, equating to $3.16 billion. Long-Term Access accounts for 28.82% or $1.50 billion, and Emergency Enteral Access makes up 10.5% at $0.55 billion, indicating a strong emphasis on various access types tailored to patient needs throughout their treatment.
Infection Prevention Enteral Access Market Analysis By End User
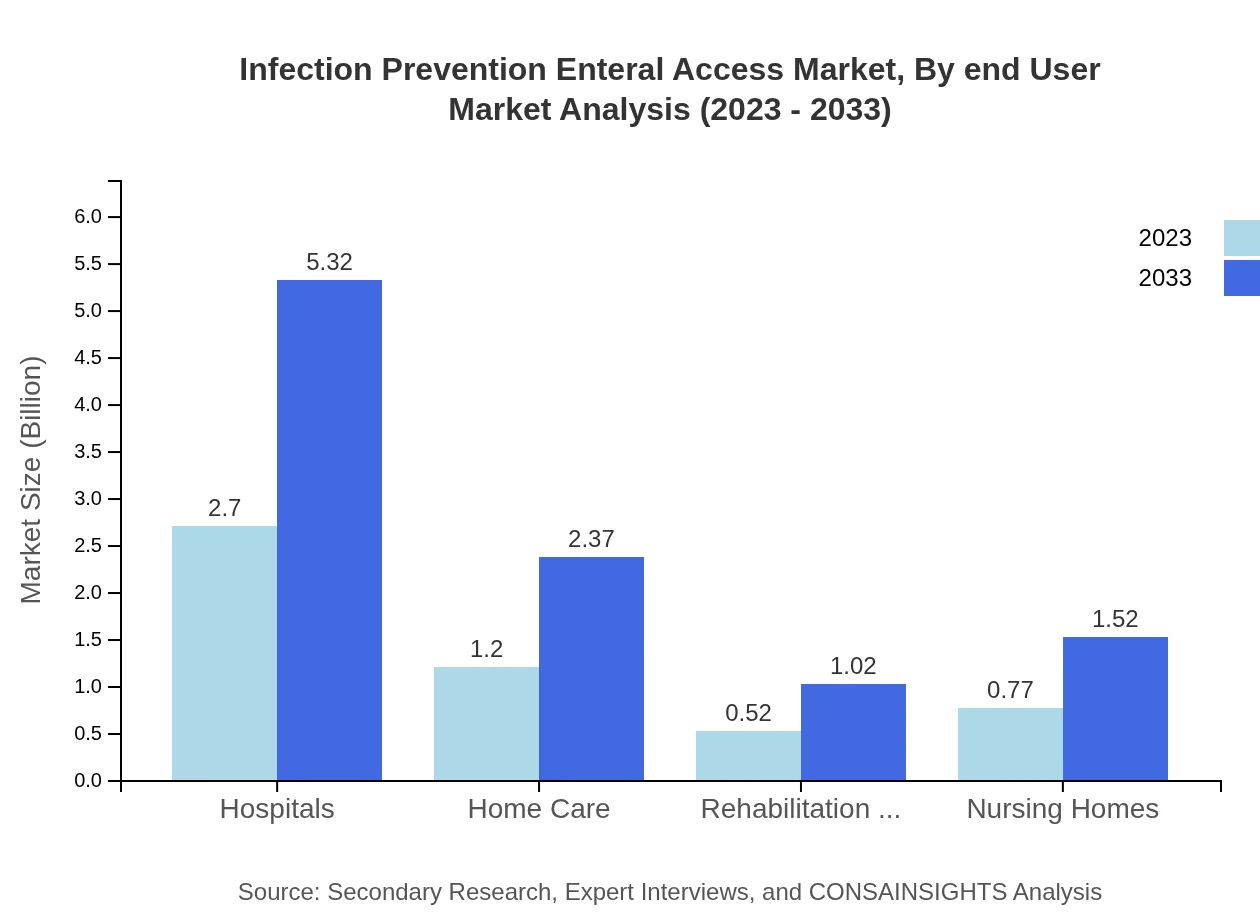
Hospitals account for the largest segment of the Infection Prevention Enteral Access market with a share of 52.01%, valued at $2.70 billion in 2023. Home Care follows with a significant 23.16% share, amounting to $1.20 billion, while Nursing Homes and Rehabilitation Centers represent 14.83% and 10%, respectively, demonstrating the diversified applications across various care environments.
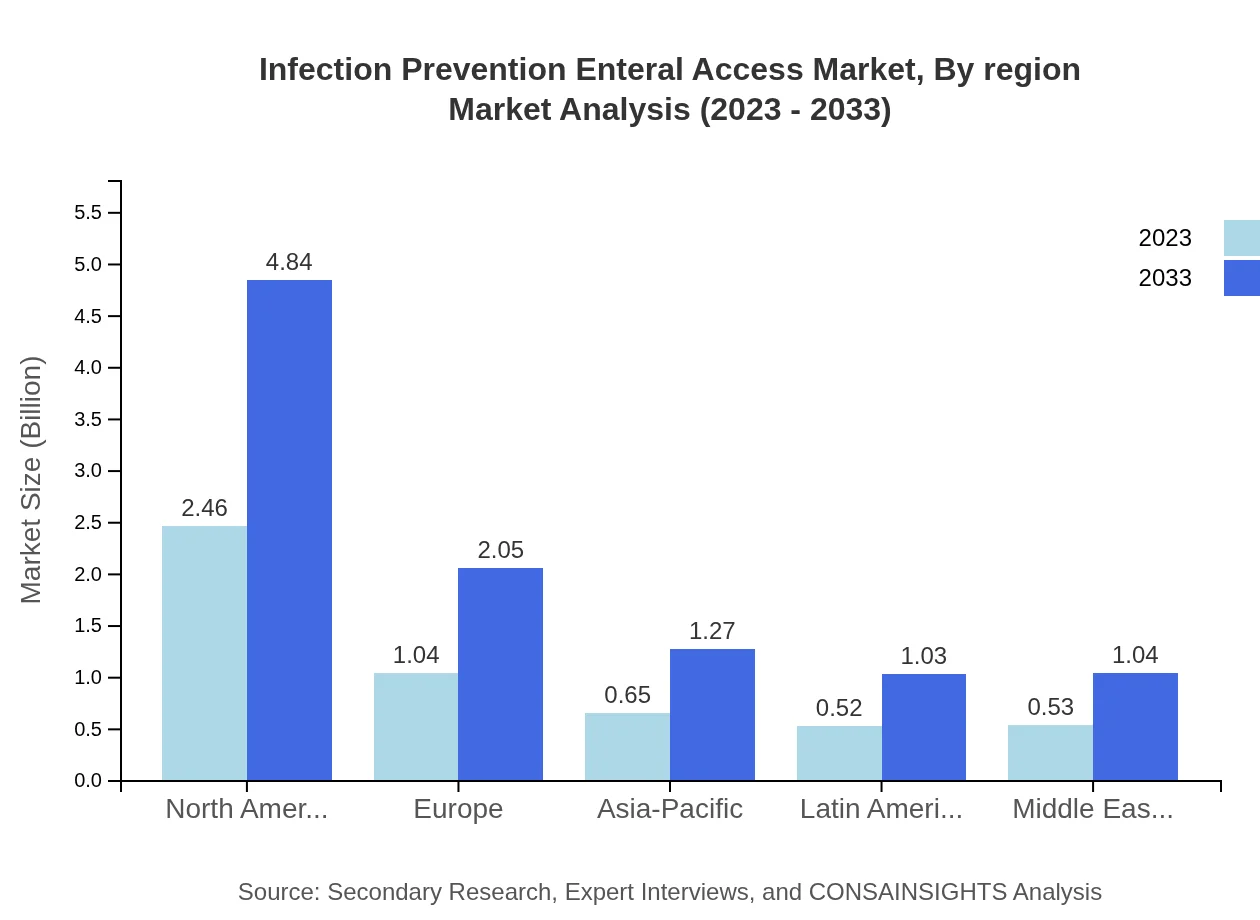
Infection Prevention Enteral Access Market Analysis By Region
Regional analysis shows that North America leads the market with a size of $2.46 billion in 2023 and a consistent share of 47.34%. Europe follows with $1.04 billion at 20.03%. Asia-Pacific stands at $0.65 billion, holding a share of 12.42%, while Latin America and the Middle East & Africa contribute $0.52 billion (10.07%) and $0.53 billion (10.14%), respectively, highlighting a rapidly growing global outlook.
Infection Prevention Enteral Access Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Infection Prevention Enteral Access Industry
Abbott Laboratories:
A leading global healthcare company specializing in medical devices, Abbott provides innovative enteral feeding products and infection prevention solutions that enhance patient care.Medtronic :
Medtronic offers a comprehensive range of enteral access systems designed for effective infection prevention, significantly impacting the healthcare market with their cutting-edge technology.Boston Scientific:
Boston Scientific is recognized for its commitment to innovation in the medical device sector, particularly in enteral access and infection control technologies.Cook Medical:
Cook Medical manufactures innovative products for enteral access and infection prevention, serving healthcare providers with quality solutions to support patient safety.B. Braun Melsungen AG:
B. Braun is a global leader in medical technology and provides a wide range of enteral feeding systems aimed at enhancing infection control and improving healthcare outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of infection Prevention Enteral Access?
The infection prevention enteral access market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 6.8% through 2033, indicating strong growth in the forthcoming years.
What are the key market players or companies in this infection Prevention Enteral Access industry?
Key players in the infection prevention enteral access industry include major medical device companies, manufacturers of enteral feeding products, and healthcare providers across various regions, all contributing significantly to market dynamics.
What are the primary factors driving the growth in the infection Prevention Enteral Access industry?
Growth drivers include increasing incidences of chronic diseases requiring enteral feeding, advancing technology in enteral access devices, and the rising demand for infection prevention strategies within healthcare settings.
Which region is the fastest Growing in the infection Prevention Enteral Access?
North America is the fastest-growing region, expecting a market increase from $1.99 billion in 2023 to $3.91 billion in 2033, due to enhanced healthcare infrastructure and rising healthcare expenditure.
Does ConsaInsights provide customized market report data for the infection Prevention Enteral Access industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs within the infection prevention enteral access industry, providing comprehensive insights.
What deliverables can I expect from this infection Prevention Enteral Access market research project?
Expect key deliverables including market analysis, competitive landscape assessments, growth forecasts, regional insights, and strategic recommendations tailored to the infection prevention enteral access sector.
What are the market trends of infection Prevention Enteral Access?
Current trends include increasing adoption of innovative enteral devices, focus on patient safety and infection control, and rising investments in healthcare technology to enhance care standards.