Infectious Disease Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: infectious-disease-therapeutics
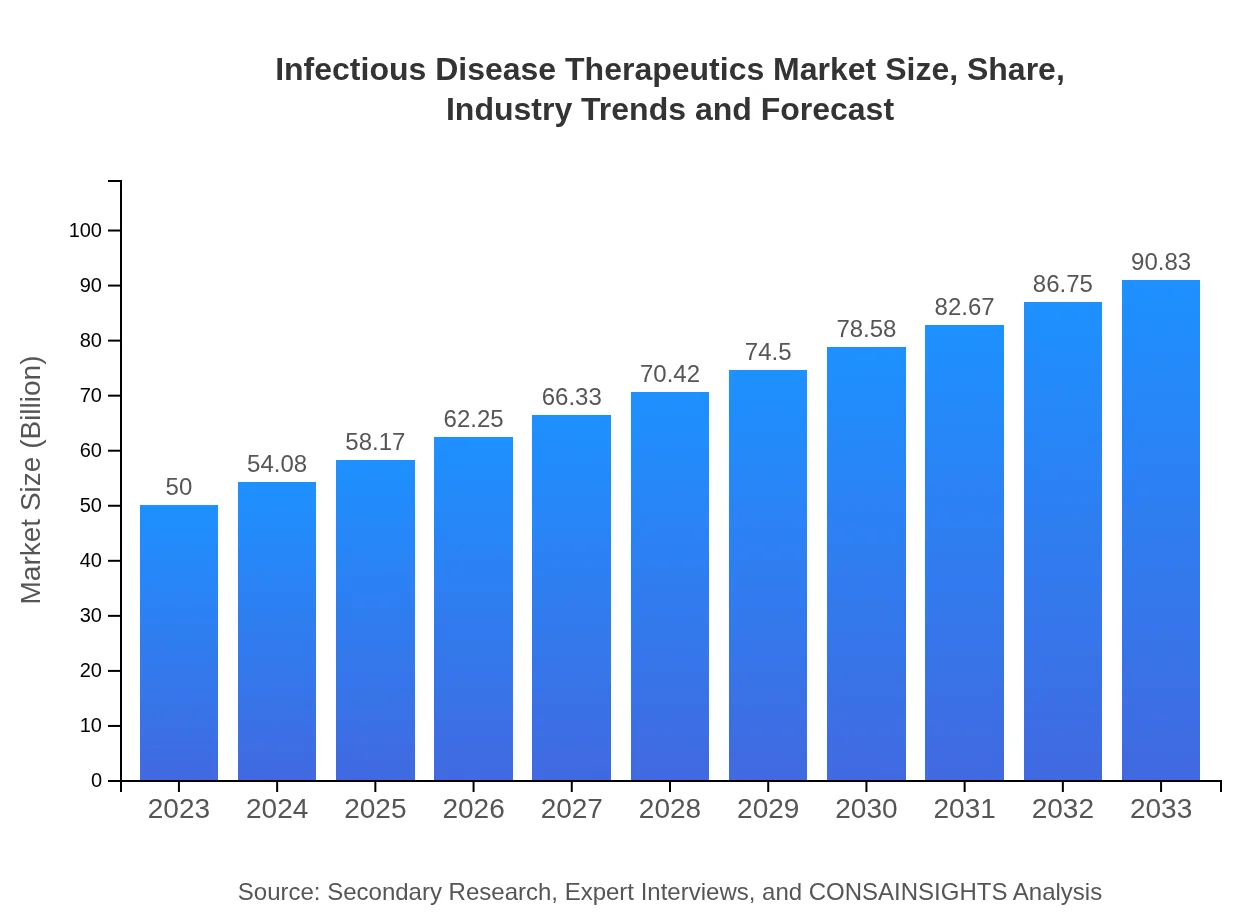
Infectious Disease Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Infectious Disease Therapeutics market, providing insights on market size, trends, and growth forecasts from 2023 to 2033. It presents an in-depth analysis of regional insights, industry challenges, and competitive landscapes.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $50.00 Billion |
| CAGR (2023-2033) | 6% |
| 2033 Market Size | $90.83 Billion |
| Top Companies | Pfizer Inc., Novartis International AG, Johnson & Johnson, Gilead Sciences, Inc. |
| Last Modified Date | 31 January 2026 |
Infectious Disease Therapeutics Market Overview
Customize Infectious Disease Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Infectious Disease Therapeutics market size, growth, and forecasts.
- ✔ Understand Infectious Disease Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Infectious Disease Therapeutics
What is the Market Size & CAGR of Infectious Disease Therapeutics market in 2023?
Infectious Disease Therapeutics Industry Analysis
Infectious Disease Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Infectious Disease Therapeutics Market Analysis Report by Region
Europe Infectious Disease Therapeutics Market Report:
Europe's market stood at $14.19 billion in 2023, anticipated to grow to $25.77 billion by 2033. Rising government initiatives for research and development, particularly for antibiotic resistance and viral diseases, support market expansion.Asia Pacific Infectious Disease Therapeutics Market Report:
In the Asia Pacific region, the Infectious Disease Therapeutics market was valued at $9.59 billion in 2023 and is projected to reach $17.41 billion by 2033. The increasing burden of infectious diseases in the region, alongside growing pharmaceutical investments, contributes to this growth. Countries like India and China prioritize developing healthcare infrastructure to combat these diseases.North America Infectious Disease Therapeutics Market Report:
North America remains a leading market for Infectious Disease Therapeutics, with a valuation of $18.55 billion in 2023, forecasted to reach $33.71 billion by 2033. The presence of major pharmaceutical companies and a robust healthcare framework significantly contribute to market dominance.South America Infectious Disease Therapeutics Market Report:
South America’s market was valued at $2.23 billion in 2023, expected to rise to $4.06 billion by 2033. The rise in public health initiatives and partnerships with global health organizations play a significant role in addressing infectious diseases in this region.Middle East & Africa Infectious Disease Therapeutics Market Report:
The market in the Middle East and Africa reached $5.44 billion in 2023, projected to grow to $9.88 billion by 2033. Efforts to improve healthcare standards and access to therapeutics are essential factors driving growth in this region.Tell us your focus area and get a customized research report.
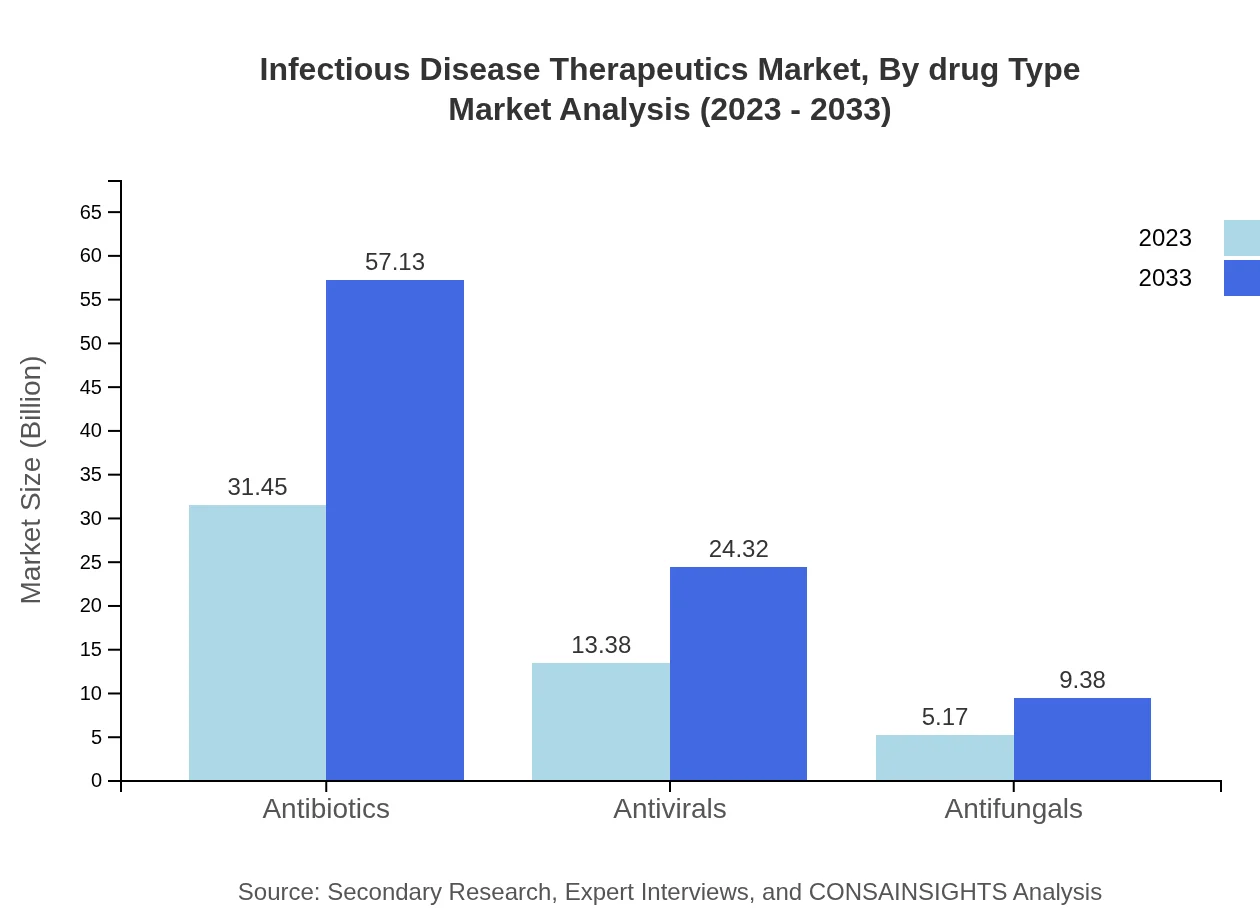
Infectious Disease Therapeutics Market Analysis By Drug Type
The Infectious Disease Therapeutics market is significantly driven by antibiotics, which hold a market share of 62.9%, valued at $31.45 billion in 2023 and expected to expand to $57.13 billion by 2033. Antivirals and antifungals also contribute notably, with markets projected to grow from $13.38 billion to $24.32 billion, and from $5.17 billion to $9.38 billion, respectively, highlighting the diversification of treatment options.
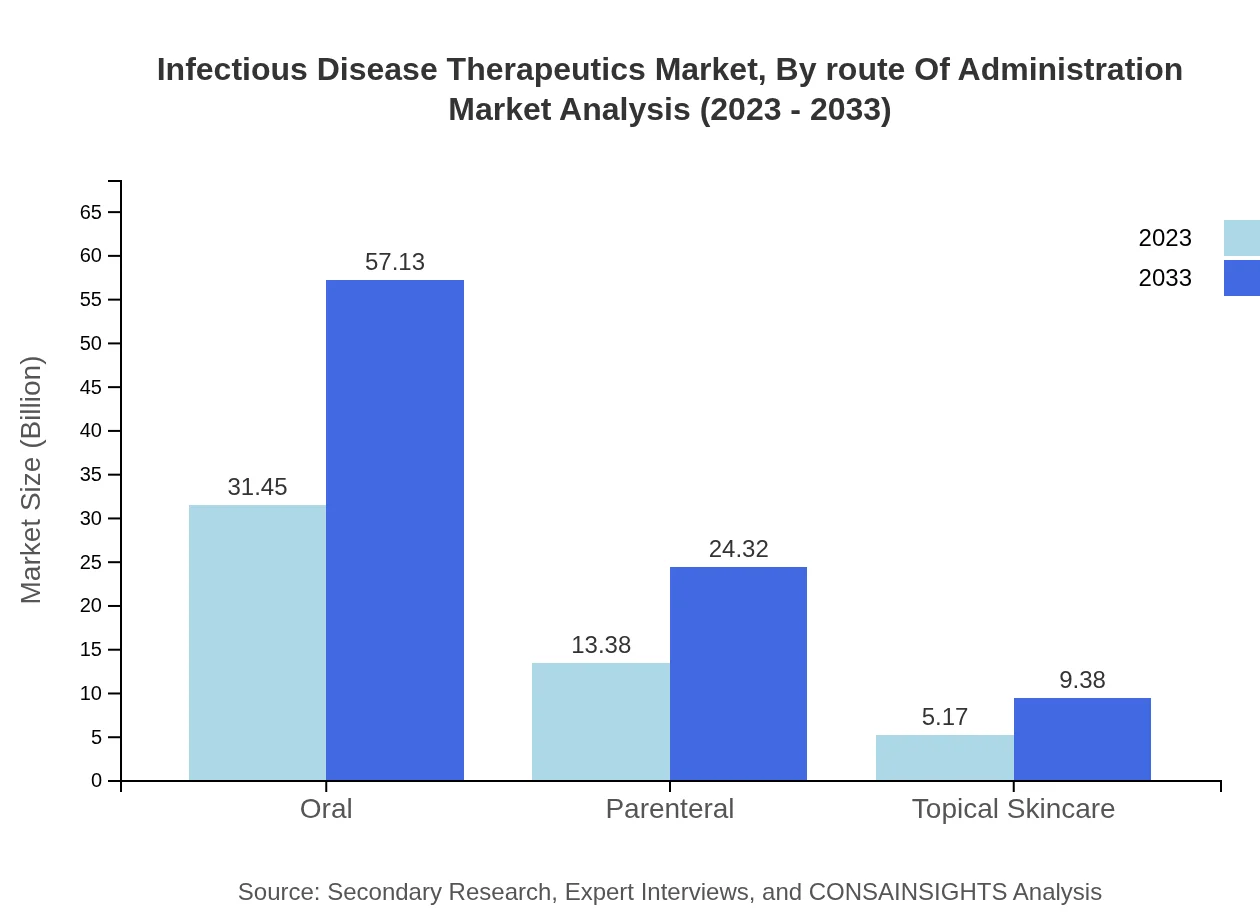
Infectious Disease Therapeutics Market Analysis By Route Of Administration
Based on route of administration, oral formulations dominate the market, holding a 62.9% market share, expanding from $31.45 billion in 2023 to $57.13 billion by 2033. Parenteral and topical forms also hold substantial shares, indicating a balanced demand across various delivery methods tailored to patient needs.
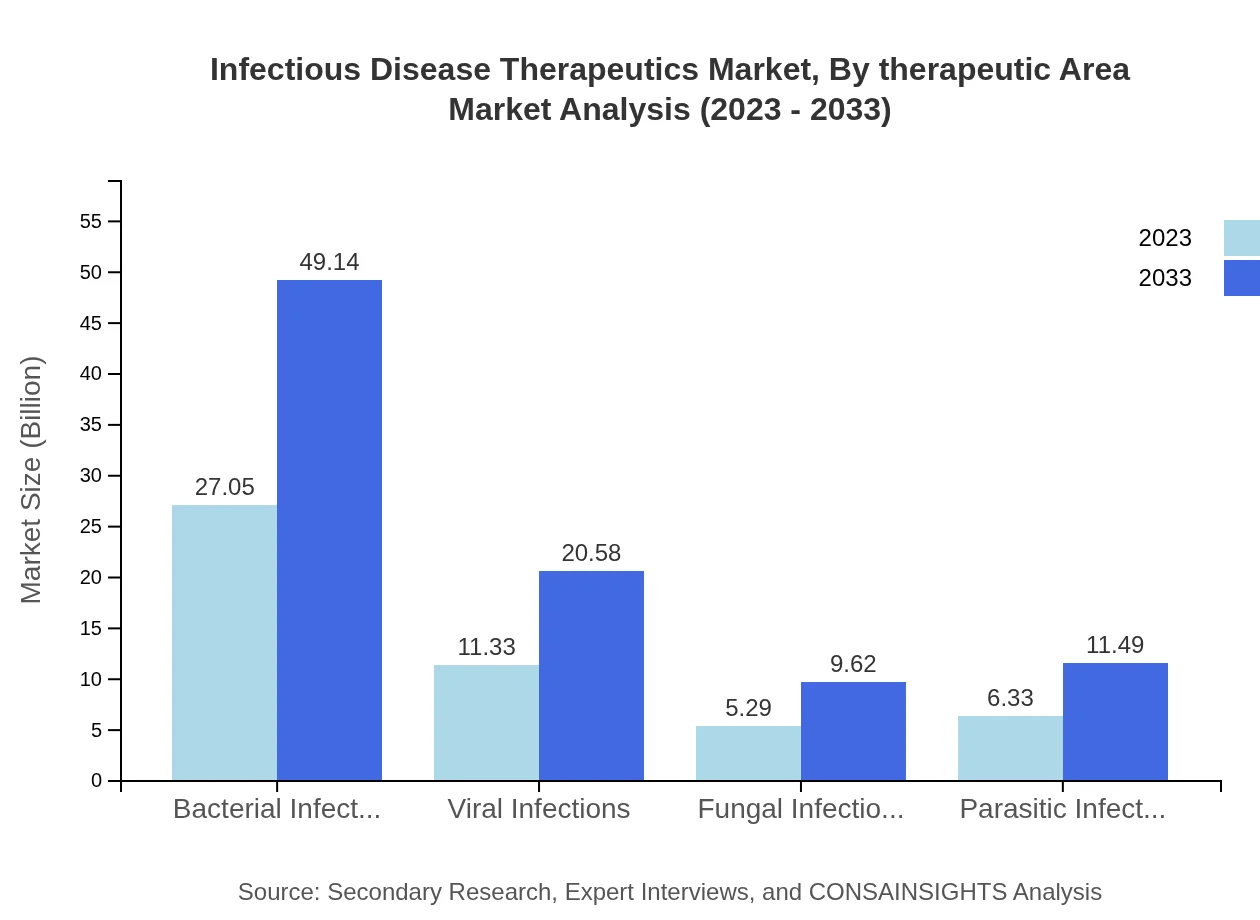
Infectious Disease Therapeutics Market Analysis By Therapeutic Area
Therapeutically, bacterial infections account for the largest segment, with a market size of $27.05 billion in 2023 and growing to $49.14 billion by 2033, representing over 54% of the total infectious disease therapeutics market. Viral infections and parasitic infections also show potential growth, emphasizing a comprehensive approach to combating infectious diseases.
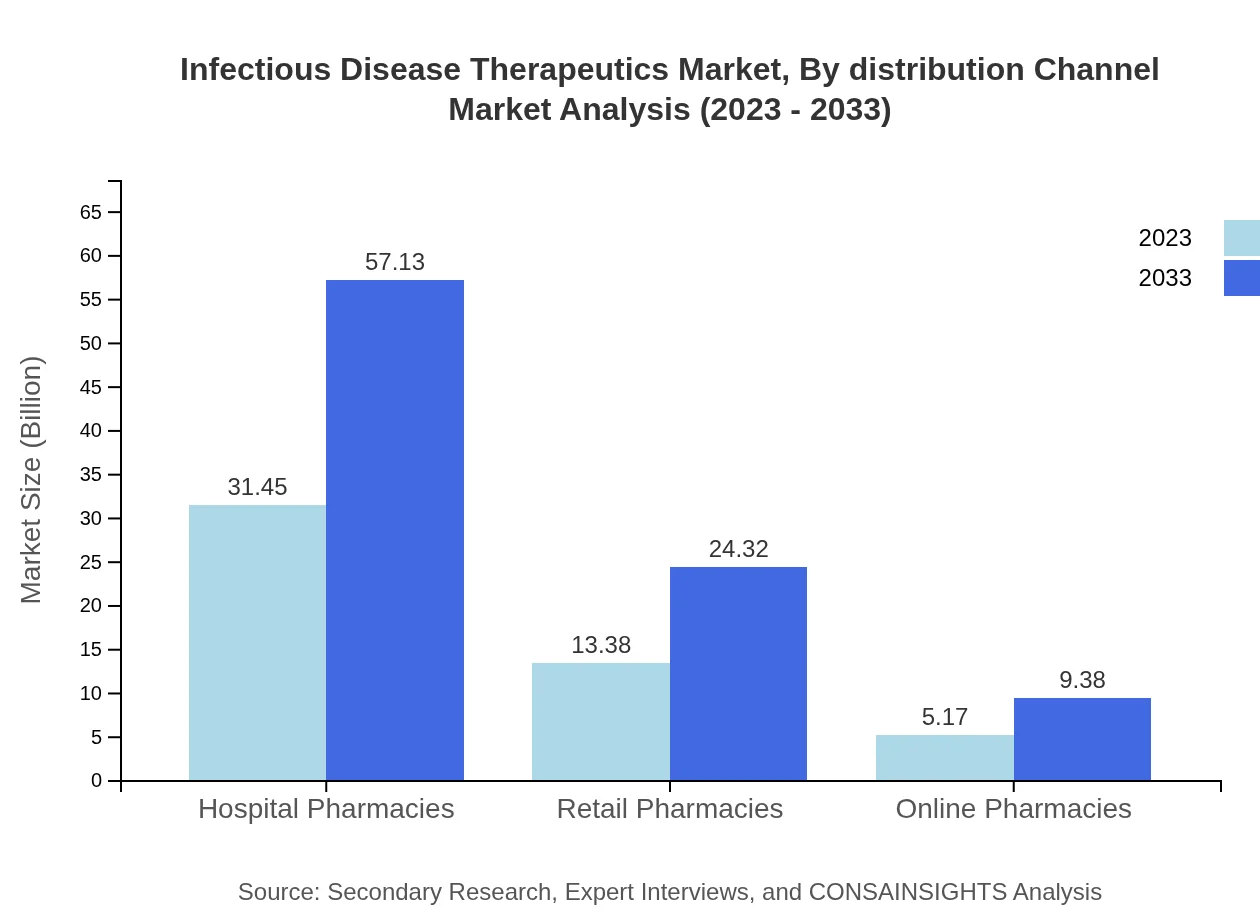
Infectious Disease Therapeutics Market Analysis By Distribution Channel
Distribution channel analysis reveals that hospital pharmacies hold a majority share, maintaining a 62.9% share with revenue growing from $31.45 billion in 2023 to $57.13 billion by 2033. Retail and online pharmacies are increasingly important, reflecting shifting consumer preferences toward accessibility and convenience.
Infectious Disease Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Infectious Disease Therapeutics Industry
Pfizer Inc.:
Pfizer is a leading pharmaceutical company, heavily involved in developing antibacterials and antivirals, including its prominent COVID-19 vaccine. The company’s research is focused on addressing antimicrobial resistance.Novartis International AG:
Novartis is a major player in the antibiotic market, known for its advanced therapeutic portfolios targeting a range of infectious diseases. The company emphasizes innovation in drug development.Johnson & Johnson:
Johnson & Johnson develops a broad spectrum of antimicrobial agents and is involved in global initiatives to enhance healthcare access, particularly in underserved regions.Gilead Sciences, Inc.:
Gilead is renowned for its antiviral drugs, particularly in treating HIV and hepatitis. The company continues to expand its portfolio to address various infectious diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of infectious Disease Therapeutics?
The infectious disease therapeutics market size is projected to reach approximately $50 billion by 2033, with a CAGR of 6% from 2023 to 2033. This growth reflects the increasing demand for effective treatment solutions in response to rising infectious diseases globally.
What are the key market players or companies in this industry?
Key players in the infectious disease therapeutics market include pharmaceutical and biotechnology firms such as Gilead Sciences, Merck & Co., and Roche. These companies are pivotal in developing innovative drugs and treatments to combat infectious diseases.
What are the primary factors driving the growth in the infectious Disease Therapeutics industry?
Growth in the infectious disease therapeutics market is driven by factors such as rising global infectious disease burden, advancements in medical technology, increased funding for research, and growing awareness and demand for effective diagnostics and treatments.
Which region is the fastest Growing in the infectious Disease Therapeutics?
North America is the fastest-growing region in the infectious disease therapeutics market, with expected growth from $18.55 billion in 2023 to $33.71 billion by 2033, reflecting a strong investment in healthcare and research initiatives.
Does ConsaInsights provide customized market report data for the infectious Disease Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the infectious disease therapeutics industry. Clients can request bespoke insights based on their unique market interests and strategic requirements.
What deliverables can I expect from this infectious Disease Therapeutics market research project?
Expected deliverables from the infectious disease therapeutics market research project include comprehensive market analysis reports, trends forecasts, competitor landscapes, segment insights, and actionable recommendations tailored to your strategic goals.
What are the market trends of infectious Disease Therapeutics?
Current trends in the infectious disease therapeutics market include increasing reliance on biologics, the rise of personalized medicine, enhanced antimicrobial stewardship, and growing investments in preventive measures, reflecting an evolving landscape in healthcare.