Inferior Vena Cava Filter Market Report
Published Date: 31 January 2026 | Report Code: inferior-vena-cava-filter
Inferior Vena Cava Filter Market Size, Share, Industry Trends and Forecast to 2033
This report presents an in-depth analysis of the Inferior Vena Cava Filter market, focusing on market trends, segmentation, technological impacts, and key drivers through 2023-2033. It provides insights into regional dynamics, product performance, and forecasts for future growth within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
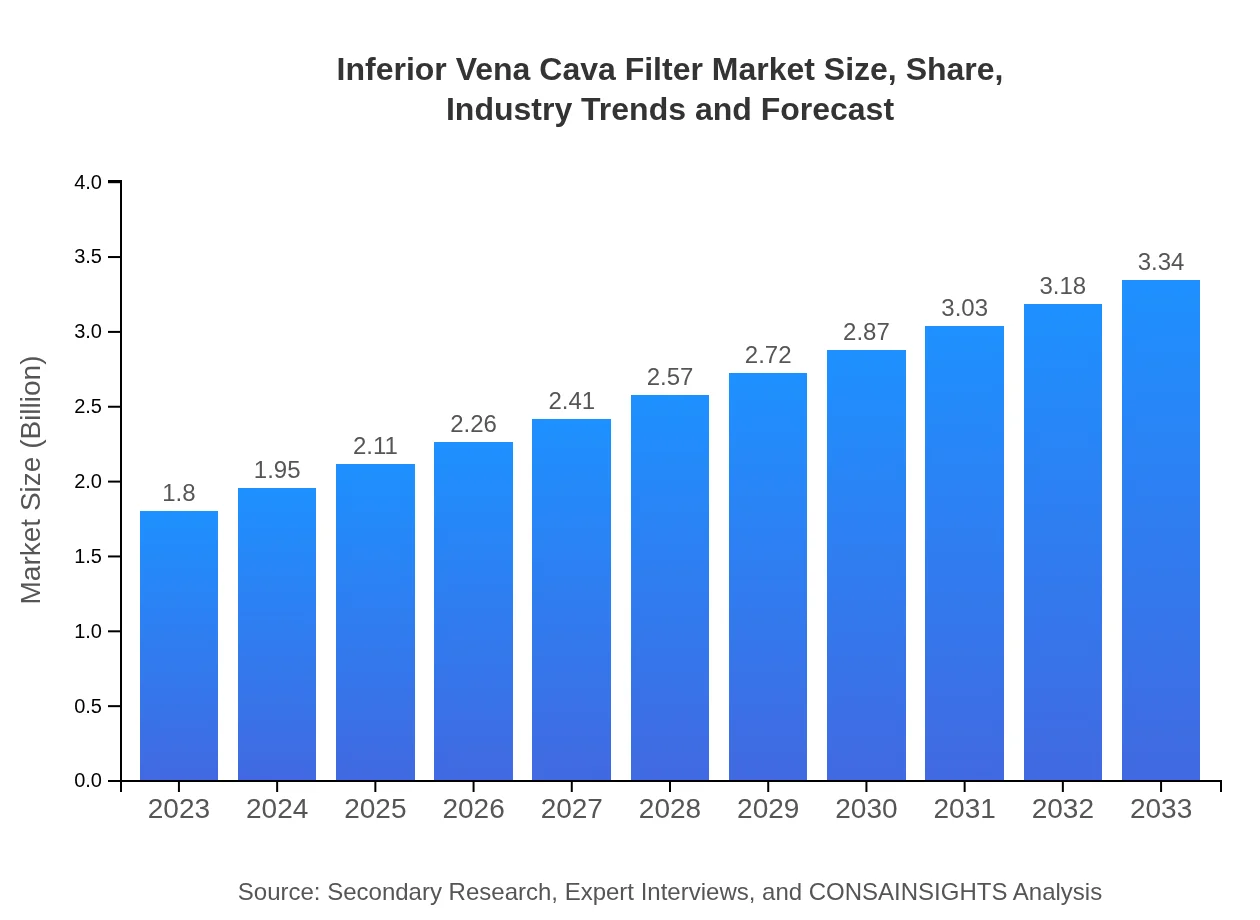
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $3.34 Billion |
| Top Companies | C.R. Bard Inc., Boston Scientific Corporation, Medtronic , Cook Medical |
| Last Modified Date | 31 January 2026 |
Inferior Vena Cava Filter Market Overview
Customize Inferior Vena Cava Filter Market Report market research report
- ✔ Get in-depth analysis of Inferior Vena Cava Filter market size, growth, and forecasts.
- ✔ Understand Inferior Vena Cava Filter's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Inferior Vena Cava Filter
What is the Market Size & CAGR of Inferior Vena Cava Filter market in 2023 and 2033?
Inferior Vena Cava Filter Industry Analysis
Inferior Vena Cava Filter Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Inferior Vena Cava Filter Market Analysis Report by Region
Europe Inferior Vena Cava Filter Market Report:
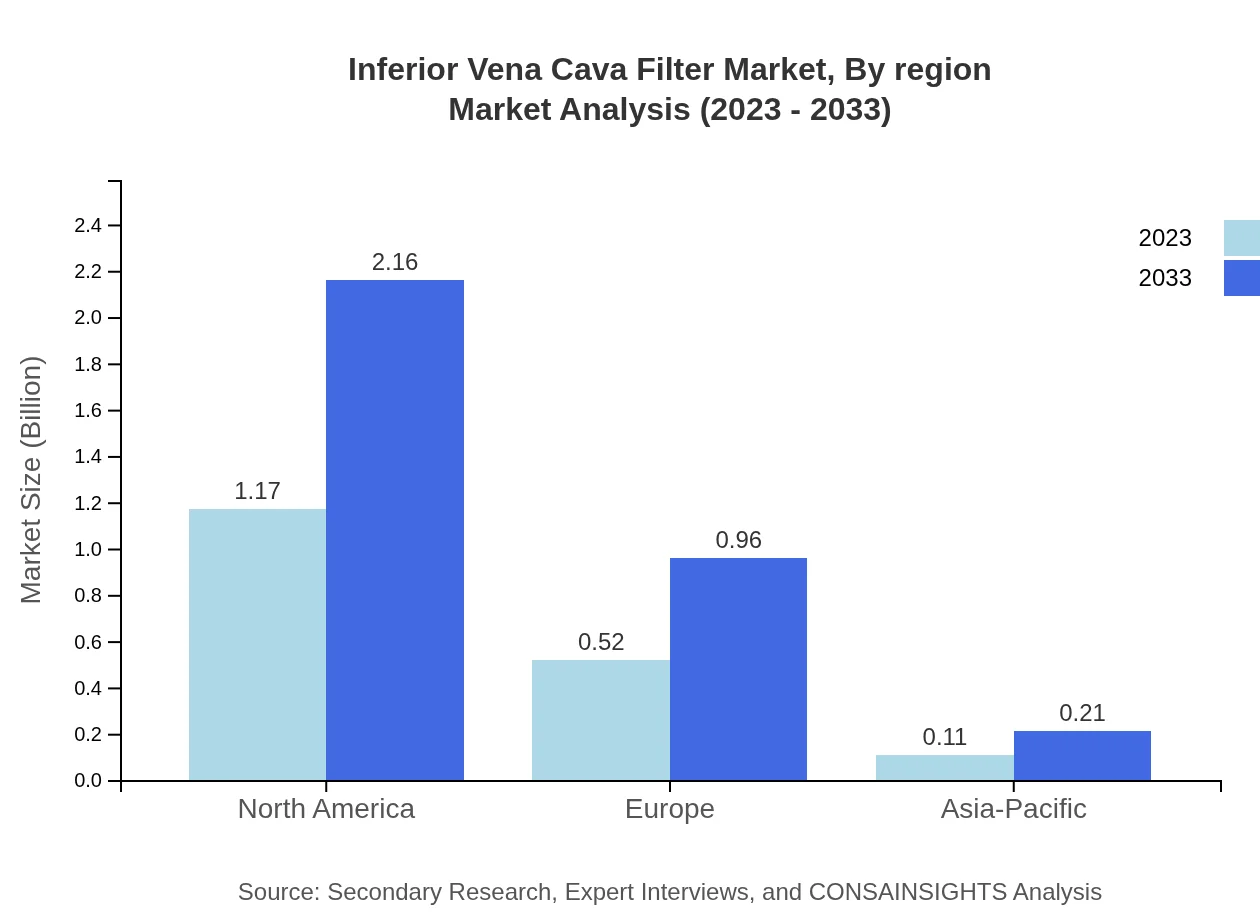
The European market is anticipated to grow from 0.47 billion USD in 2023 to 0.87 billion USD by 2033. Factors such as increasing regulatory approvals for new devices and a rise in clinical practices targeting thromboembolic disorders underscore market potential in this region.Asia Pacific Inferior Vena Cava Filter Market Report:
In Asia Pacific, the IVC filter market is estimated to grow from 0.36 billion USD in 2023 to 0.67 billion USD by 2033. Increasing adoption of advanced medical technologies and rising healthcare expenditures are key drivers for this growth, coupled with a rising geriatric population.North America Inferior Vena Cava Filter Market Report:
North America currently leads the global IVC filter market, valued at 0.70 billion USD in 2023, projected to grow to 1.29 billion USD by 2033. This growth is fueled by high prevalence rates of VTE, along with advanced healthcare infrastructure and significant investment in innovative medical devices.South America Inferior Vena Cava Filter Market Report:
The South American market, currently valued at 0.13 billion USD in 2023, is expected to reach 0.25 billion USD in 2033. Growth is primarily driven by expanding access to healthcare services and increasing cases of thrombotic diseases.Middle East & Africa Inferior Vena Cava Filter Market Report:
The Middle East and Africa market is expected to grow from 0.14 billion USD in 2023 to 0.26 billion USD by 2033, driven by improvements in healthcare infrastructure, rising awareness about thrombotic diseases, and governmental investments in healthcare.Tell us your focus area and get a customized research report.
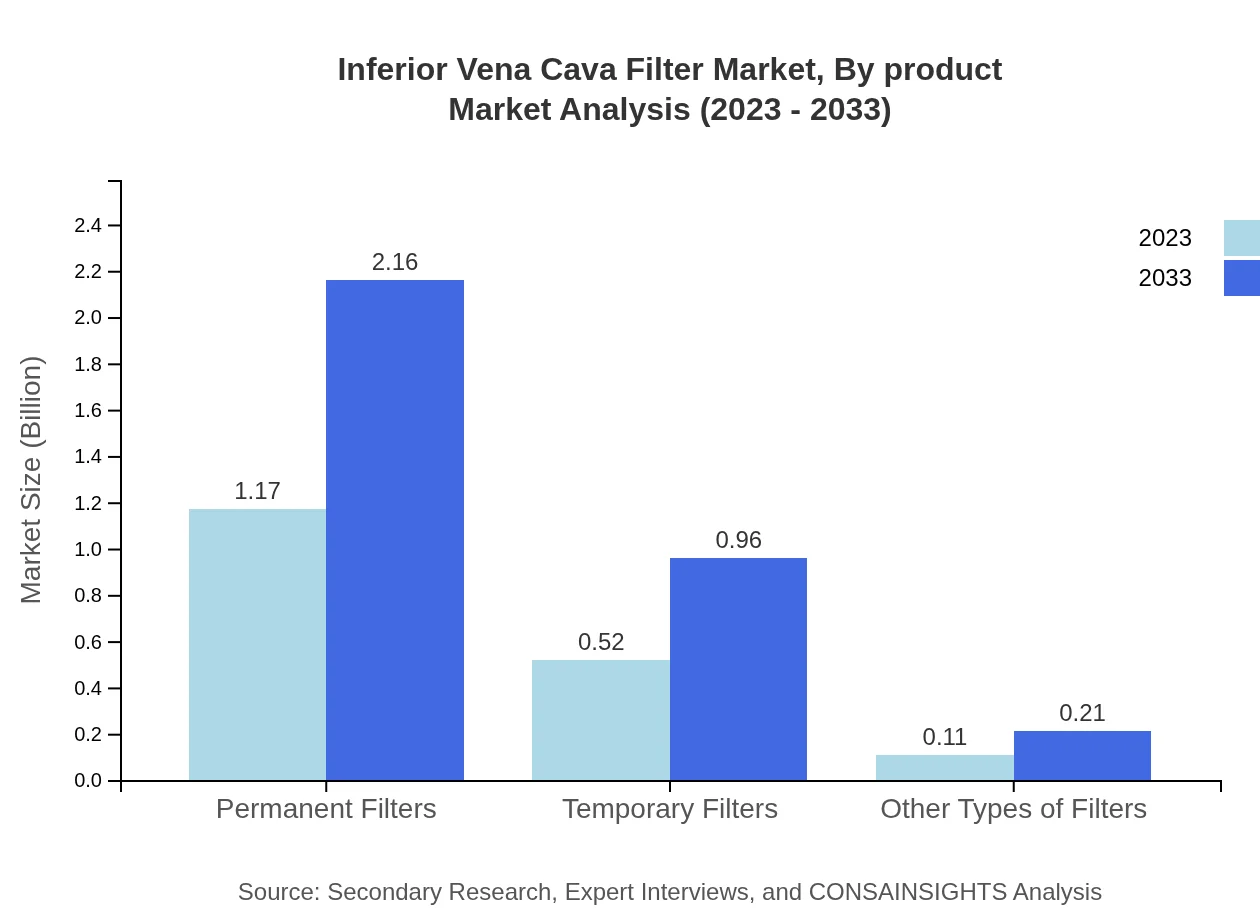
Inferior Vena Cava Filter Market Analysis By Product
The market by product type highlights Permanent Filters dominating the segment with a market size of 1.17 billion USD in 2023, projected to touch 2.16 billion USD by 2033. Temporary Filters, currently at 0.52 billion USD, are expected to increase to 0.96 billion USD. Other types, albeit smaller, exhibit growth momentum.
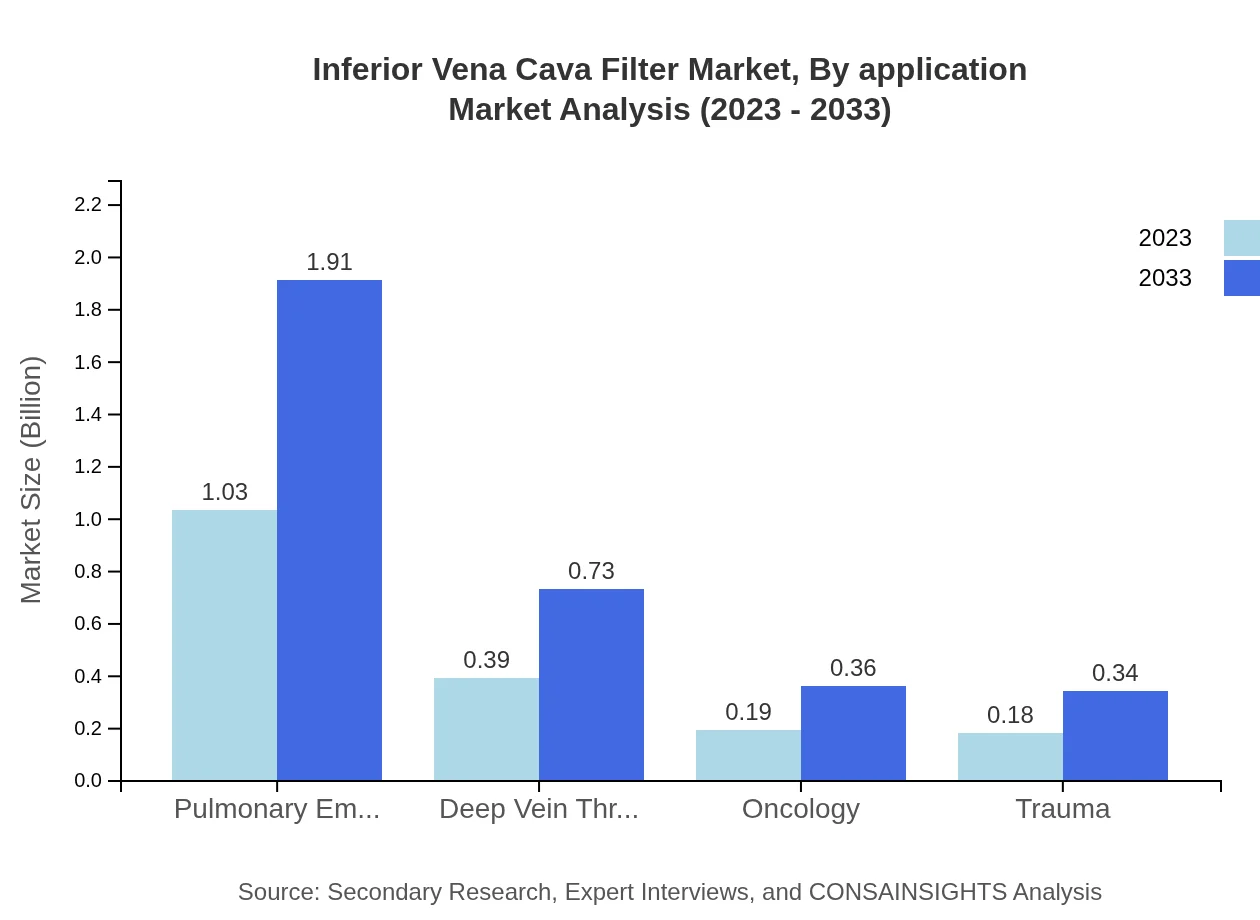
Inferior Vena Cava Filter Market Analysis By Application
Applications segmented into management of Pulmonary Embolism and Deep Vein Thrombosis, show significant growth. The Pulmonary Embolism segment leads with a 57.18% market share in 2023, anticipated to remain dominant through 2033 as awareness for public health improves.
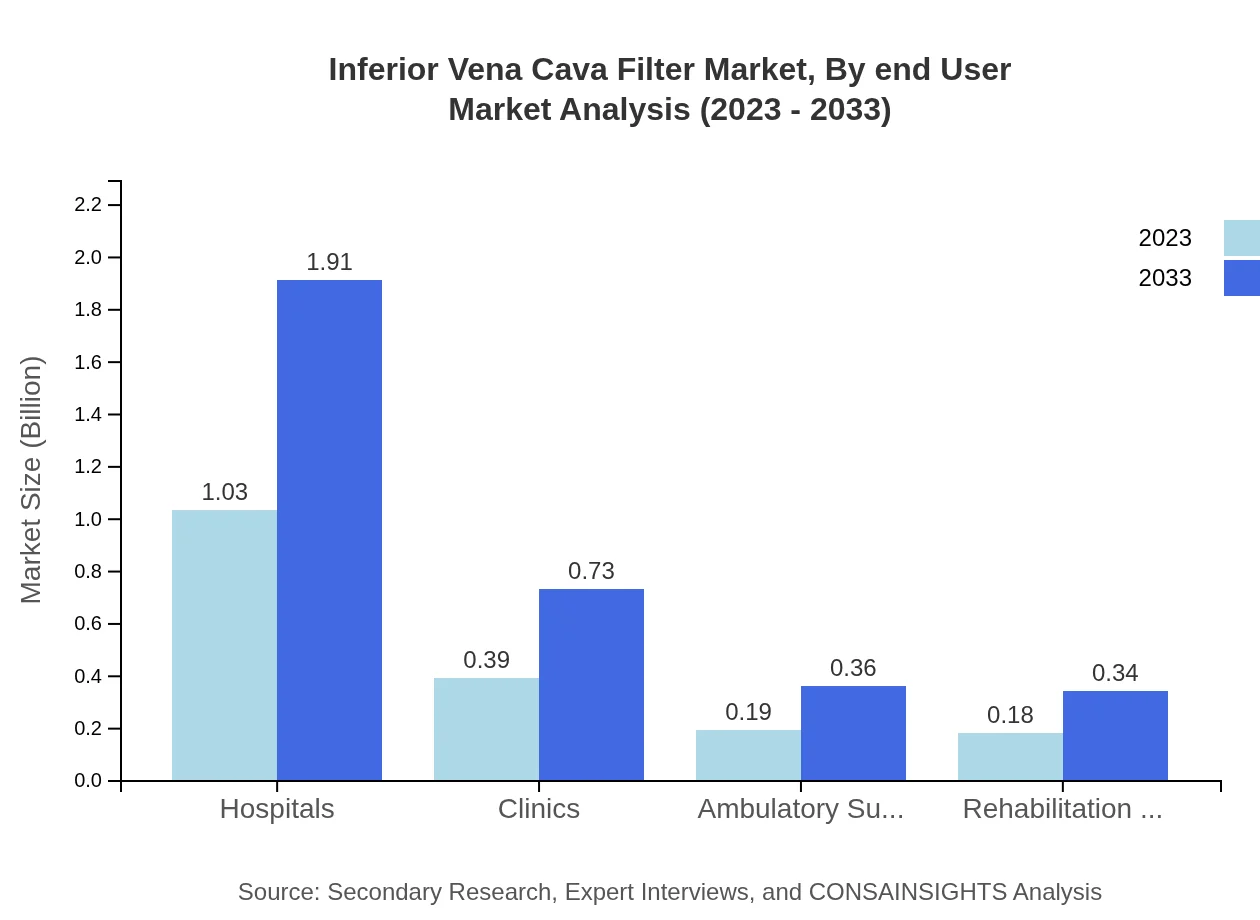
Inferior Vena Cava Filter Market Analysis By End User
Hospitals currently hold a significant 57.18% share of IVC filter use, reflecting growth driven by an increase in surgical interventions in hospitals. Clinics follow with 21.85% and Ambulatory Surgical Centers at 10.74%.
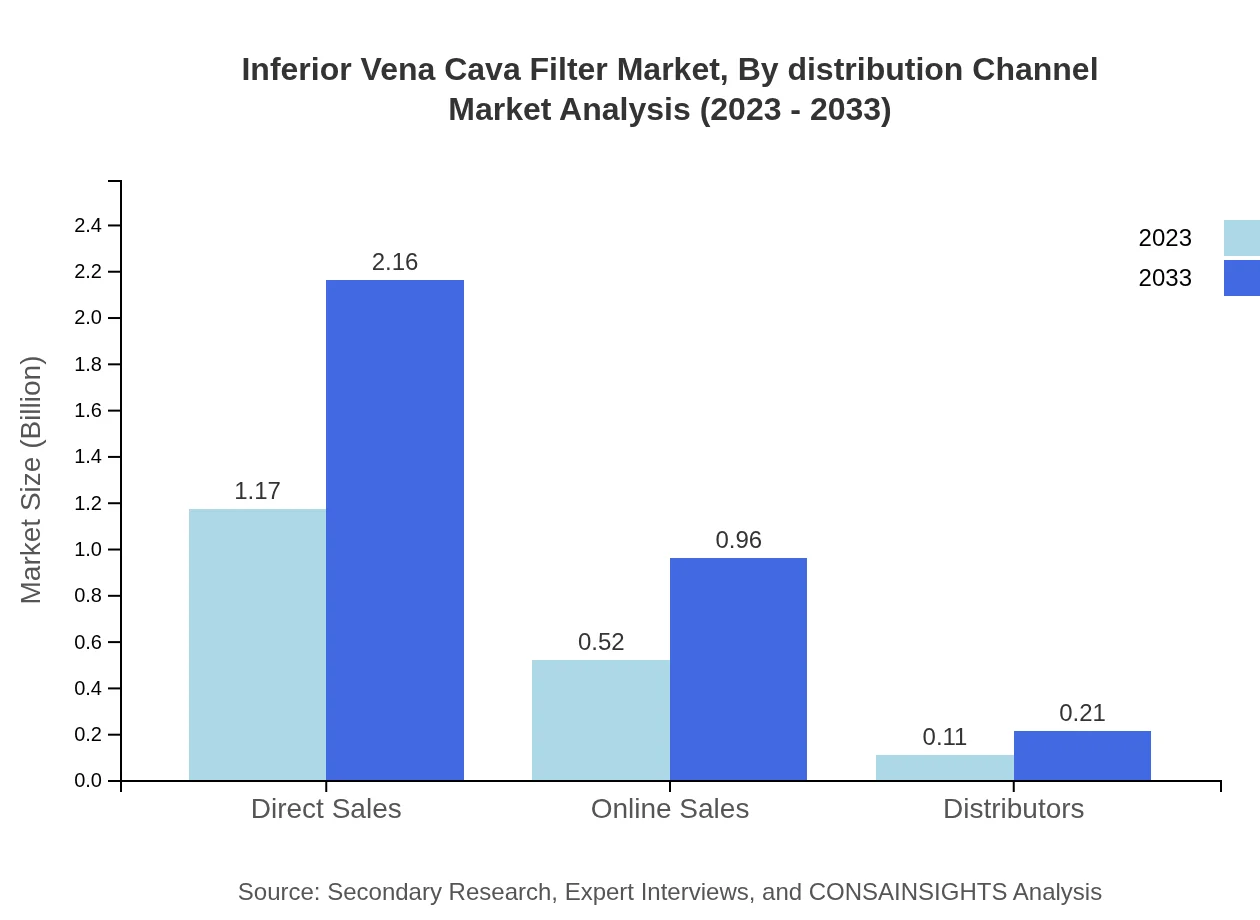
Inferior Vena Cava Filter Market Analysis By Distribution Channel
The distribution channels are primarily Direct Sales and Online Sales, with direct channels constituting about 64.81% of the market. Online sales have gained traction, indicating a shift towards e-commerce in medical device distribution.
Inferior Vena Cava Filter Market Analysis By Region
Regional analysis indicates North America as the market leader, followed by Europe and Asia Pacific. Each region's growth dynamics depend on factors such as healthcare policies, economic progress, and technological advancements.
Inferior Vena Cava Filter Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Inferior Vena Cava Filter Industry
C.R. Bard Inc.:
A pioneer in vascular access and vascular surgery, known for innovation in IVC filters focusing on safety and cost efficiency.Boston Scientific Corporation:
Key player in medical devices, leveraging technology to produce advanced IVC filter solutions aimed at reducing complication rates.Medtronic :
Leader in medical technology offering a range of vascular solutions including prominent products in the IVC filter market.Cook Medical:
Involved extensively in vascular intervention, focusing on stakeholder collaboration to innovate evidence-based solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of inferior Vena Cava Filter?
The Inferior Vena Cava Filter market is currently valued at approximately $1.8 billion, with a projected CAGR of 6.2% from 2023 to 2033, indicating a robust growth trajectory.
What are the key market players or companies in this inferior Vena Cava Filter industry?
Key players in the Inferior Vena Cava Filter market include leading companies focused on vascular devices and medical technologies. Their innovations are essential for maintaining competitive advantage in this growing market.
What are the primary factors driving the growth in the inferior Vena Cava Filter industry?
Key factors driving market growth include increasing prevalence of venous thromboembolism, advances in filter technology, and a growing emphasis on preventive care in healthcare systems worldwide.
Which region is the fastest Growing in the inferior Vena Cava Filter?
North America currently leads the market while also projected to grow significantly, reaching $1.29 billion by 2033. The Asia-Pacific region is also rapidly expanding, expected to reach $0.67 billion.
Does ConsaInsights provide customized market report data for the inferior Vena Cava Filter industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs and queries regarding the Inferior Vena Cava Filter industry, ensuring comprehensive insights for stakeholders.
What deliverables can I expect from this inferior Vena Cava Filter market research project?
Expect detailed market analyses, forecasts, competitive landscape insights, segmentation data, and trend evaluations that provide a strategic overview of the Inferior Vena Cava Filter market.
What are the market trends of inferior Vena Cava Filter?
Current trends in the Inferior Vena Cava Filter market include the rise of permanent filters, shifting focus towards minimally invasive procedures, and increased usage in hospitals and clinics.