Influenza Diagnostics Market Report
Published Date: 31 January 2026 | Report Code: influenza-diagnostics
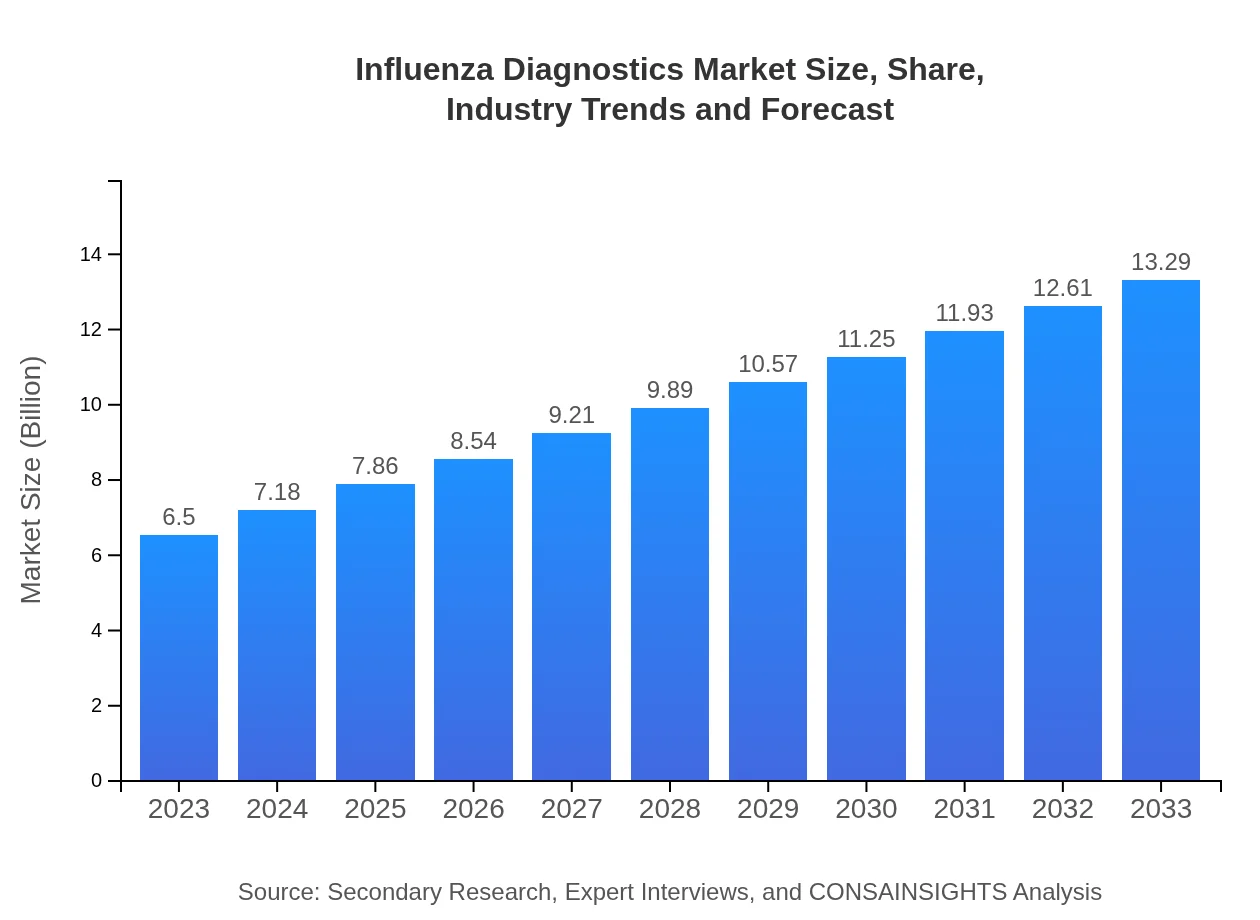
Influenza Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report covers a comprehensive analysis of the Influenza Diagnostics market, including key insights, market trends, and forecasts from 2023 to 2033. It presents data on market size, regional dynamics, technology innovations, and industry leaders driving future growth.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $6.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $13.29 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Cepheid, Becton, Dickinson and Company, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Influenza Diagnostics Market Overview
Customize Influenza Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Influenza Diagnostics market size, growth, and forecasts.
- ✔ Understand Influenza Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Influenza Diagnostics
What is the Market Size & CAGR of Influenza Diagnostics market in 2023?
Influenza Diagnostics Industry Analysis
Influenza Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Influenza Diagnostics Market Analysis Report by Region
Europe Influenza Diagnostics Market Report:
In Europe, the market size is expected to start at $1.96 billion in 2023 and double to $4.01 billion by 2033. Increasing incidences of influenza and initiatives to improve diagnostic practices are significant contributors.Asia Pacific Influenza Diagnostics Market Report:
In 2023, the Influenza Diagnostics market in the Asia Pacific region is estimated at $1.22 billion, with expectations to grow to $2.49 billion by 2033. The market is driven by increasing healthcare investments, rising awareness regarding influenza screening, and technological enhancements in diagnosis.North America Influenza Diagnostics Market Report:
North America will dominate the Influenza Diagnostics market with a size of $2.40 billion in 2023, growing to $4.91 billion by 2033. The growth is supported by robust healthcare infrastructure, advanced diagnostics capabilities, and heightened awareness of public health.South America Influenza Diagnostics Market Report:
The South American Influenza Diagnostics market is projected to reach $0.34 billion in 2023, with growth to $0.70 billion anticipated by 2033. Key factors include improving access to healthcare services and an uptick in diagnostic testing adoption.Middle East & Africa Influenza Diagnostics Market Report:
The market in the Middle East and Africa is projected at $0.58 billion in 2023, with a growth forecast to $1.18 billion by 2033. Increased health investments and awareness about influenza's impact are driving growth in this region.Tell us your focus area and get a customized research report.
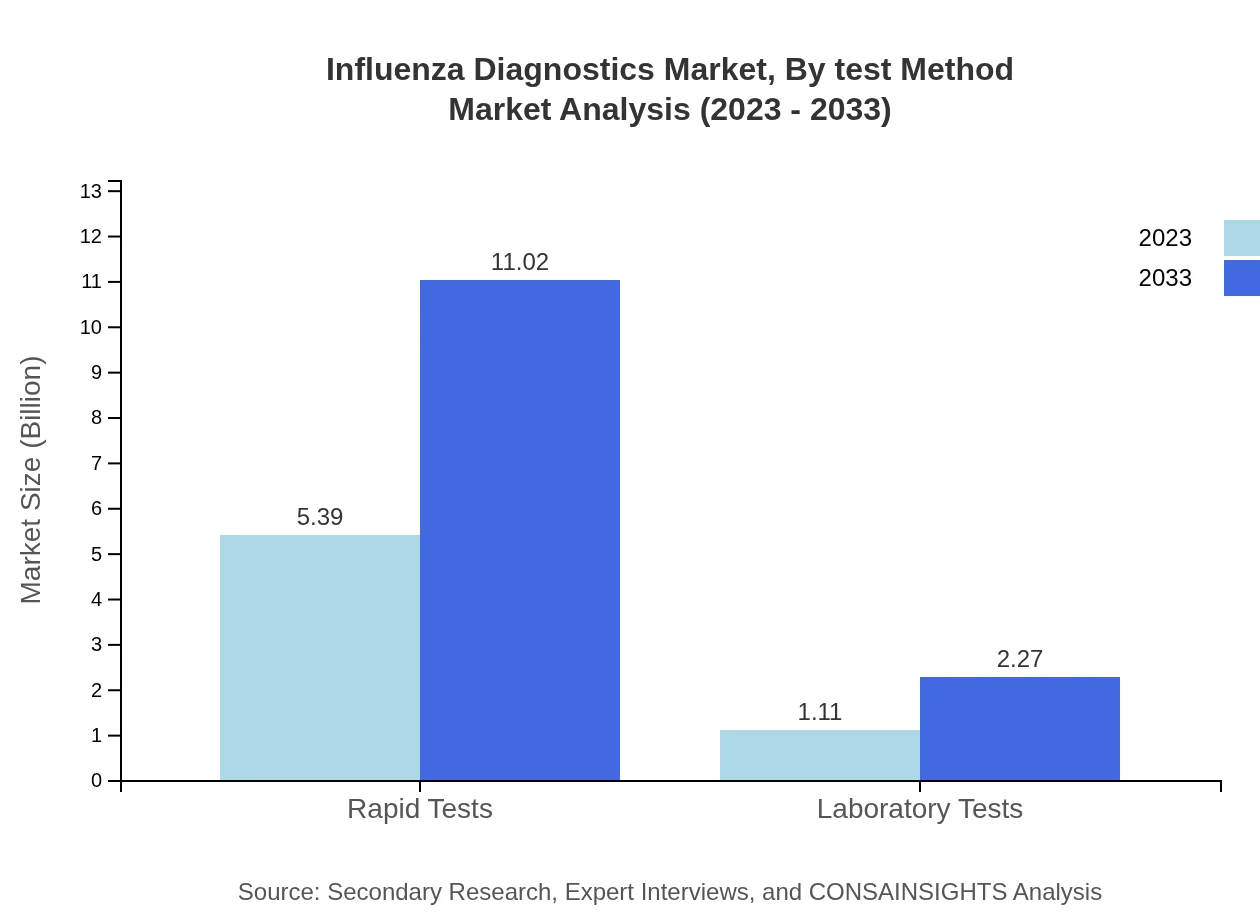
Influenza Diagnostics Market Analysis By Test Method
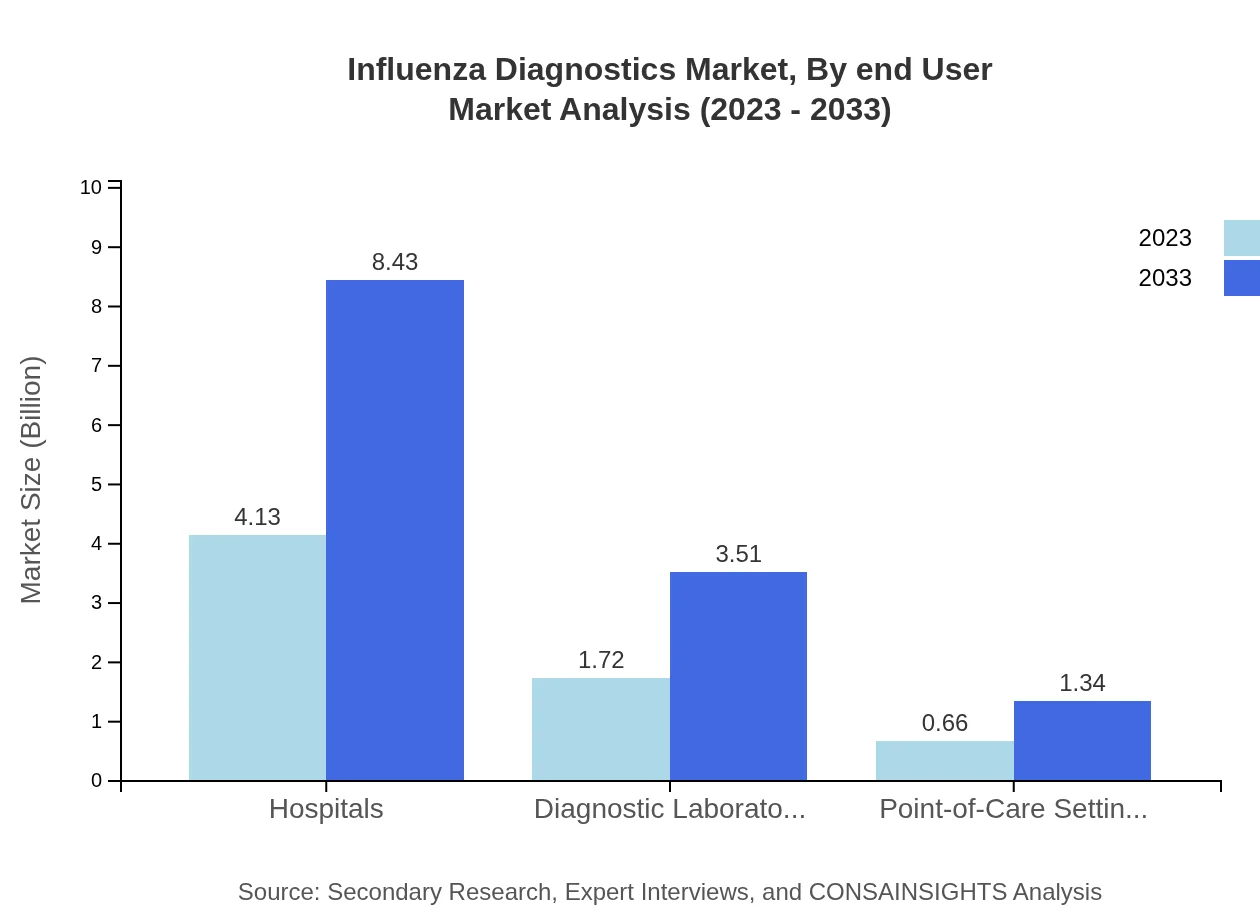
The market is greatly influenced by demographics in healthcare settings. Hospitals lead with a market size of $4.13 billion in 2023, expected to reach $8.43 billion by 2033, retaining a 63.48% market share. Diagnostic laboratories follow with $1.72 billion in 2023 and $3.51 billion by 2033, accounting for 26.43%. Point-of-care settings are gaining traction as rapid testing becomes increasingly vital, forecasting $0.66 billion growth to $1.34 billion by 2033.
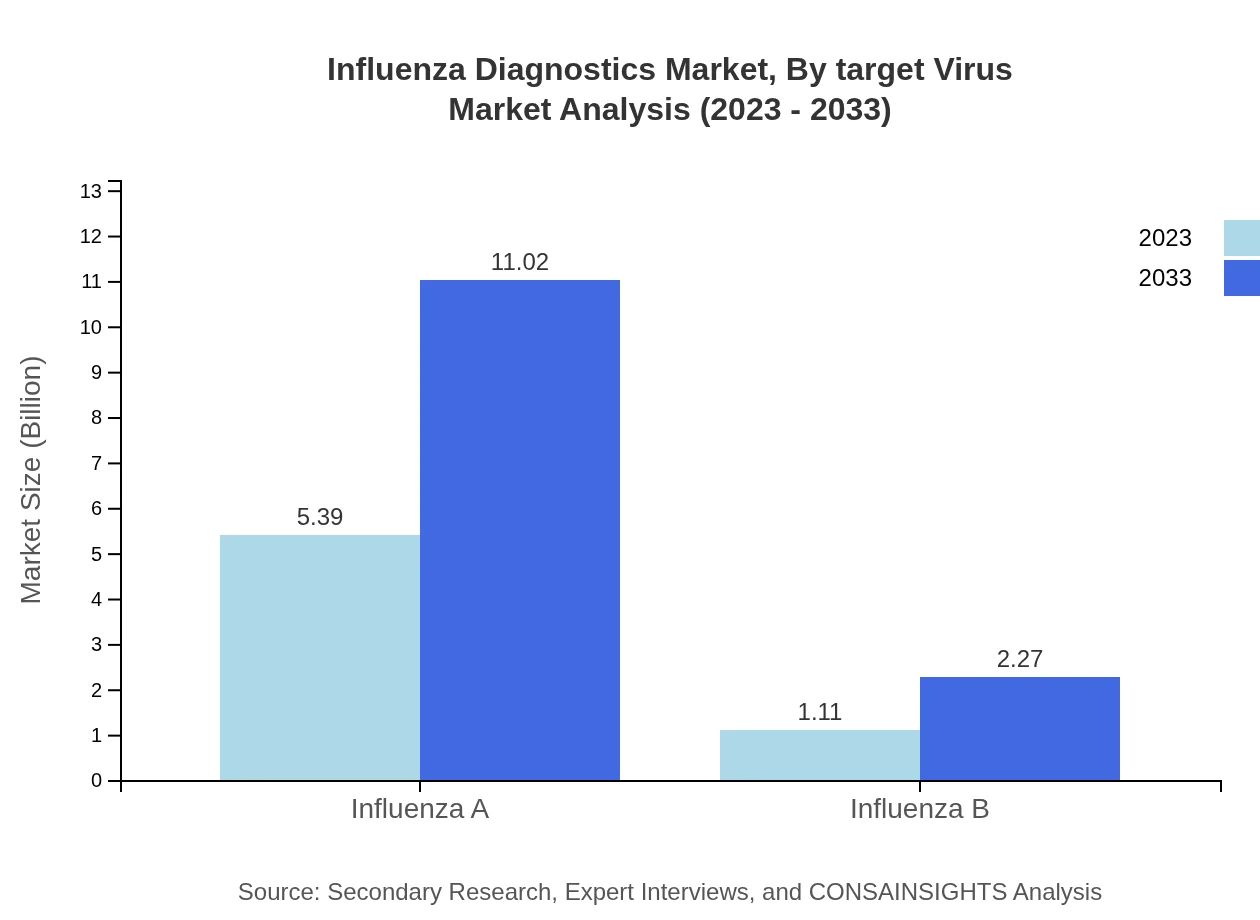
Influenza Diagnostics Market Analysis By Target Virus
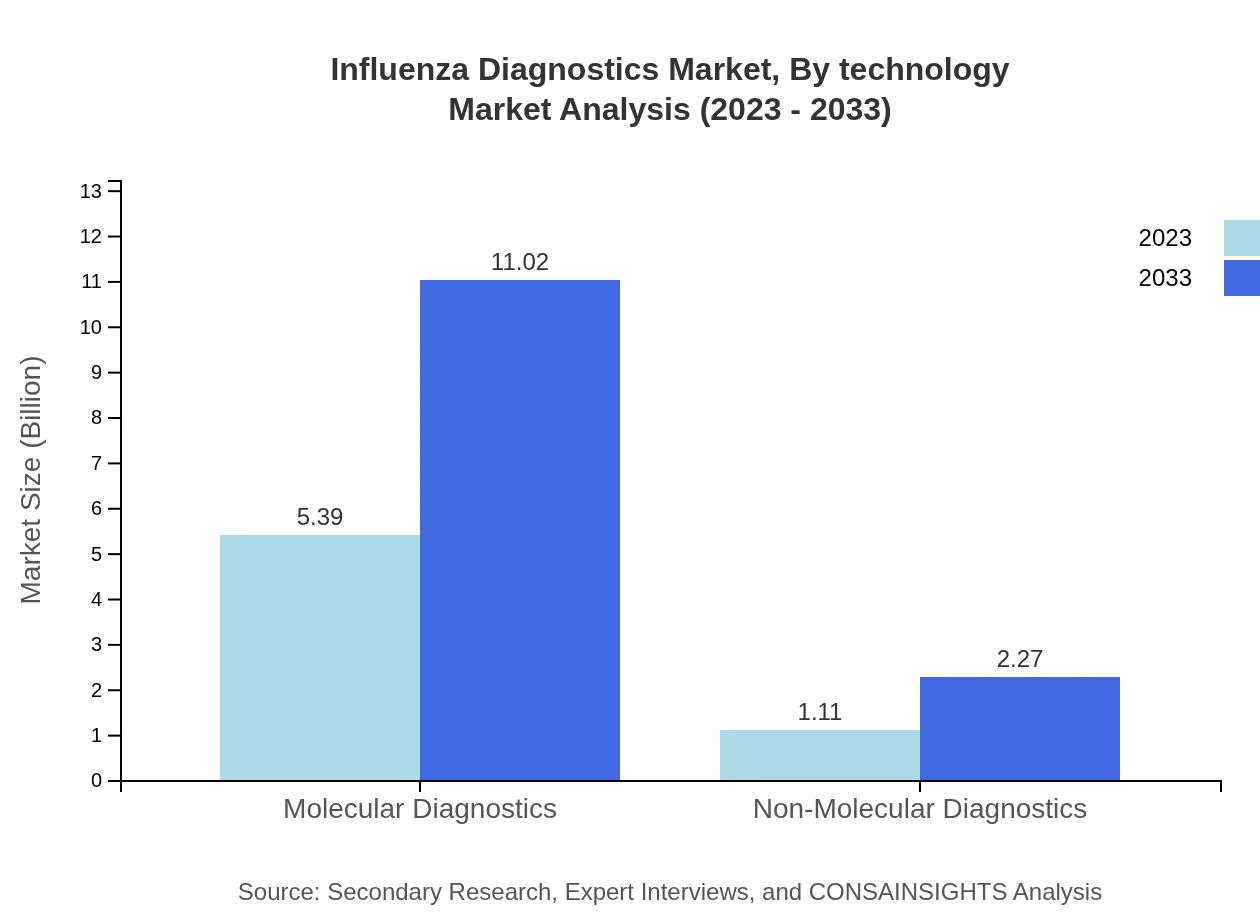
Molecular diagnostics dominate the market with a size of $5.39 billion in 2023 projected to rise to $11.02 billion by 2033, holding an impressive 82.92% share. Non-molecular diagnostics are gaining ground, with a forecast from $1.11 billion in 2023 to $2.27 billion by 2033, representing 17.08%.
Influenza Diagnostics Market Analysis By Technology
The market shows considerable reliance on rapid tests which account for $5.39 billion in 2023 and are expected to reach $11.02 billion by 2033 with an 82.92% market share. Laboratory tests represent a significant segment as well with growth projections reflecting the need for comprehensive diagnostic strategies.
Influenza Diagnostics Market Analysis By End User
Hospitals are the leading end-users of influenza diagnostics, solidifying their essential role in managing influenza outbreaks. With a market share of 63.48%, hospitals are projected to sustain the largest sector share through 2033, contrasted by diagnostic laboratories and point-of-care settings that cater to diversifying healthcare needs.
Influenza Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Influenza Diagnostics Industry
Abbott Laboratories:
A leading player known for its rapid molecular diagnostics, Abbott’s innovative systems have revolutionized influenza testing processes.Roche Diagnostics:
Roche’s advanced diagnostics solutions, focusing on accuracy and reliability, continue to set a high standard in the influenza testing domain.Cepheid:
Specializing in molecular diagnostics, Cepheid’s revolutionary rapid tests have propelled the company to the forefront of the influenza diagnostics market.Becton, Dickinson and Company:
BD’s extensive portfolio of diagnostic products and commitment to innovation enables it to address a wide range of influenza diagnostic needs effectively.Thermo Fisher Scientific:
With a focus on cutting-edge laboratory equipment and diagnostics, Thermo Fisher plays a crucial role in enhancing influenza testing accuracy.We're grateful to work with incredible clients.









FAQs
What is the market size of influenza Diagnostics?
The global Influenza Diagnostics market is projected to reach a size of approximately $6.5 billion by 2033, growing at a CAGR of 7.2% from 2023. This growth is driven by increasing demand for accurate diagnostic tools and enhanced healthcare infrastructure.
What are the key market players or companies in this influenza Diagnostics industry?
Key players in the influenza diagnostics market include established companies like Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, and BD. These firms are at the forefront of developing advanced diagnostic technologies tailored to manage influenza outbreaks effectively.
What are the primary factors driving the growth in the influenza diagnostics industry?
Several factors contribute to the growth of the influenza diagnostics industry. Increased incidences of influenza, rising awareness about contagious diseases, advancements in diagnostic technologies, and supportive government initiatives drive demand for accurate and timely diagnosis.
Which region is the fastest Growing in the influenza diagnostics?
The Asia-Pacific region is the fastest-growing market for influenza diagnostics, expected to reach $2.49 billion by 2033. Rapid urbanization, improved healthcare facilities, and rising healthcare expenditure bolster market growth in this region.
Does ConsaInsights provide customized market report data for the influenza diagnostics industry?
Yes, ConsaInsights offers customized market report data for the influenza diagnostics industry, allowing businesses to gain insights tailored to their specific needs and market conditions. Customized reports help stakeholders make informed decisions based on detailed analyses.
What deliverables can I expect from this influenza diagnostics market research project?
Deliverables from the influenza diagnostics market research project will typically include comprehensive market analysis reports, segment data, trend analysis, competitive landscape assessments, regional data insights, and actionable recommendations for market entry and growth strategies.
What are the market trends of influenza diagnostics?
Current market trends in influenza diagnostics include the growing shift towards rapid test kits, increasing adoption of molecular diagnostics, heightened demand for point-of-care testing, and a focus on integrating advanced technologies such as AI for enhanced diagnostic accuracy.