Influenza Market Report
Published Date: 31 January 2026 | Report Code: influenza
Influenza Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Influenza market from 2023 to 2033, covering market size, growth trends, industry dynamics, key market players, and forecasts. Insights on regional performance, technology advancements, and product analysis are also included.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
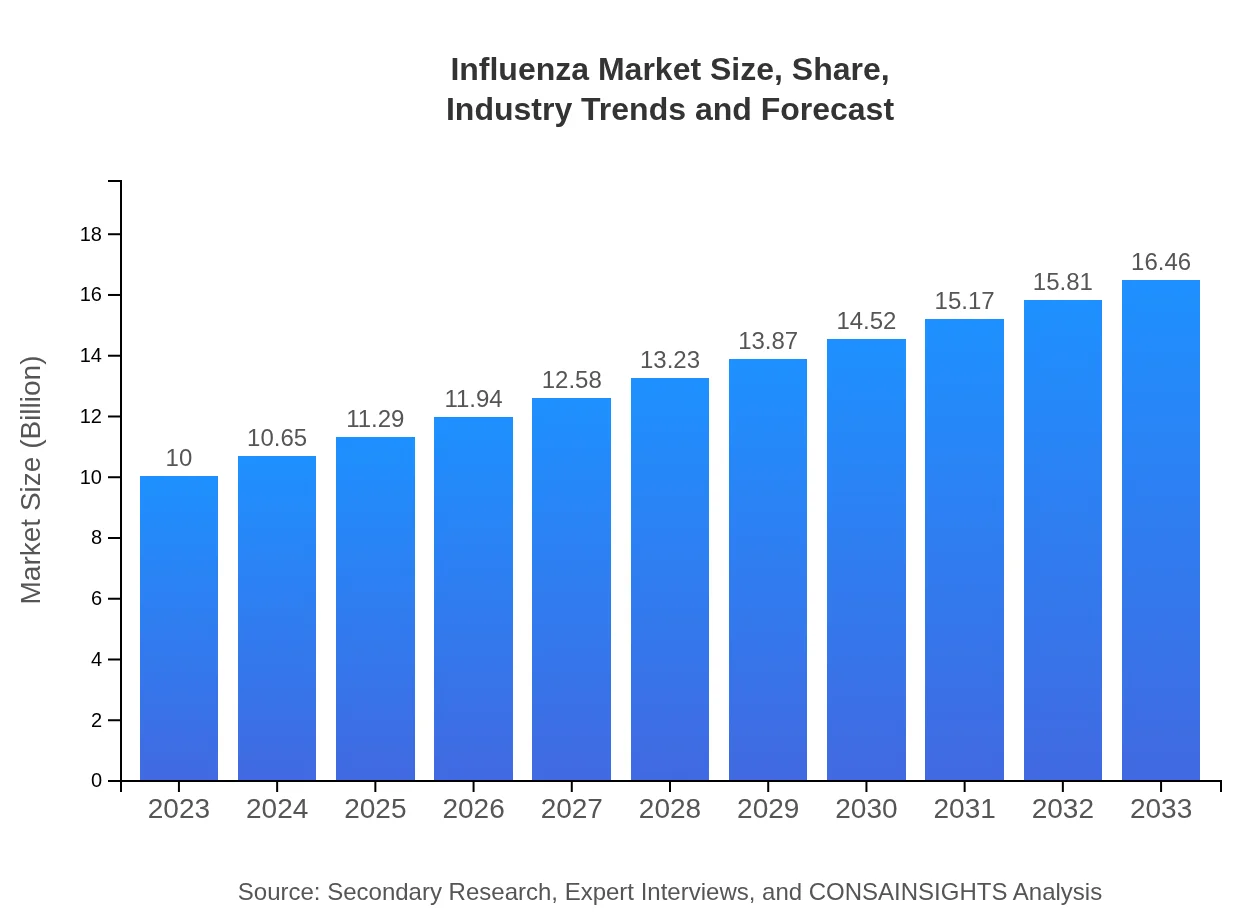
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | Sanofi Pasteur, GlaxoSmithKline (GSK), Pfizer Inc., Novartis AG, Merck & Co., Inc. |
| Last Modified Date | 31 January 2026 |
Influenza Market Overview
Customize Influenza Market Report market research report
- ✔ Get in-depth analysis of Influenza market size, growth, and forecasts.
- ✔ Understand Influenza's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Influenza
What is the Market Size & CAGR of Influenza market in 2023?
Influenza Industry Analysis
Influenza Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Influenza Market Analysis Report by Region
Europe Influenza Market Report:
In Europe, the market is expected to rise from $2.62 billion in 2023 to $4.32 billion by 2033. The emphasis on preventative healthcare and government policies promoting vaccination heavily influence growth. Increasing healthcare awareness and innovative vaccine technologies also play crucial roles.Asia Pacific Influenza Market Report:
In the Asia Pacific region, the Influenza market is projected to grow from $2.13 billion in 2023 to $3.51 billion by 2033. The growth is driven by increasing healthcare expenditures, enhanced vaccination campaigns, and a rising population. Countries like China and India are focusing on improving public health measures to combat influenza outbreaks.North America Influenza Market Report:
North America represents a substantial market, growing from $3.40 billion in 2023 to $5.59 billion by 2033. The high prevalence of seasonal influenza, coupled with strong healthcare reimbursement policies and an active pharmaceutical sector, supports this expansion. The U.S. and Canada are major contributors to this growth.South America Influenza Market Report:
The South American market is set to increase from $0.68 billion in 2023 to $1.11 billion in 2033, spurred by improving healthcare infrastructure and vaccination rates. Regional governments are heavily investing in vaccination campaigns, leading to increased awareness and adoption.Middle East & Africa Influenza Market Report:
The Middle East and Africa market will experience growth from $1.18 billion in 2023 to $1.94 billion by 2033. Growing healthcare facilities in emerging economies and increasing awareness about influenza prevention drive this market, although challenges such as accessibility remain.Tell us your focus area and get a customized research report.
Influenza Market Analysis By Type
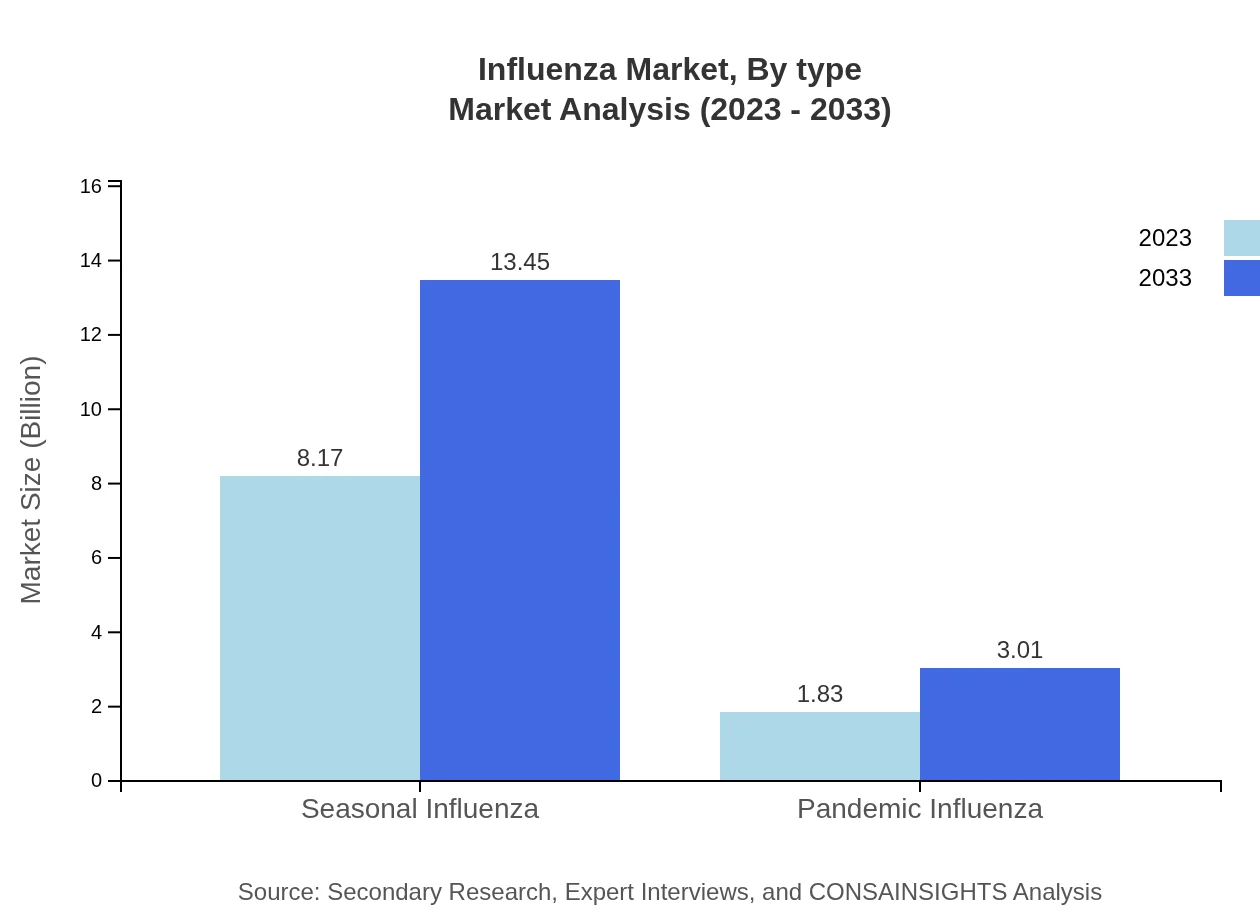
The Influenza market can be divided into two primary categories: Seasonal Influenza and Pandemic Influenza. Seasonal Influenza comprises the larger share of the market, growing from $8.17 billion in 2023 to $13.45 billion by 2033, maintaining a strong 81.72% market share. The Pandemic Influenza segment, although smaller, is significant, expected to rise from $1.83 billion in 2023 to $3.01 billion in 2033, holding an 18.28% market share.
Influenza Market Analysis By Vaccine Type
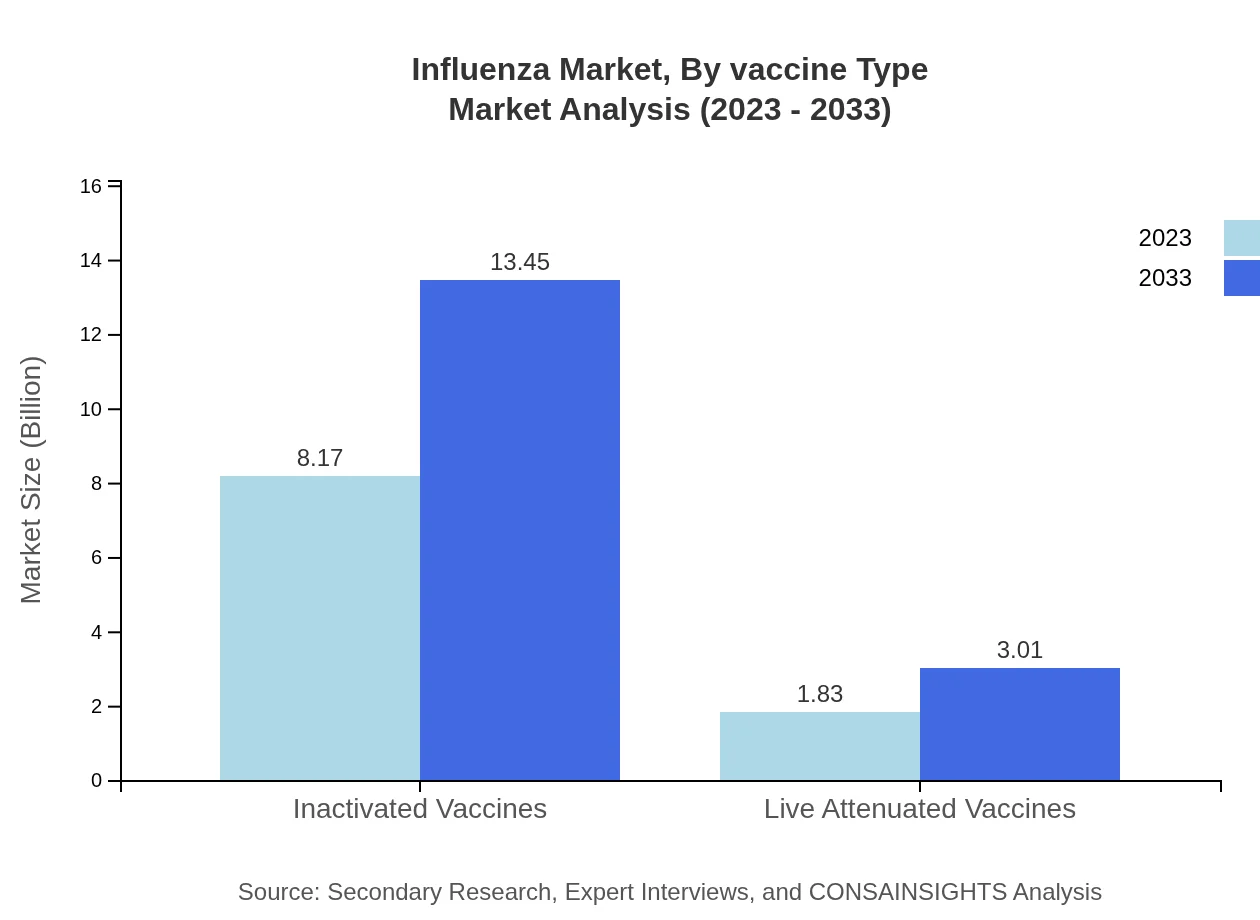
Vaccine types are categorized into Inactivated and Live Attenuated Vaccines. Inactivated Vaccines dominate the market, growing from $8.17 billion in 2023 to $13.45 billion by 2033, maintaining an 81.72% share. Live Attenuated Vaccines are also significant, growing from $1.83 billion in 2023 to $3.01 billion by 2033, comprising an 18.28% share.
Influenza Market Analysis By Distribution Channel
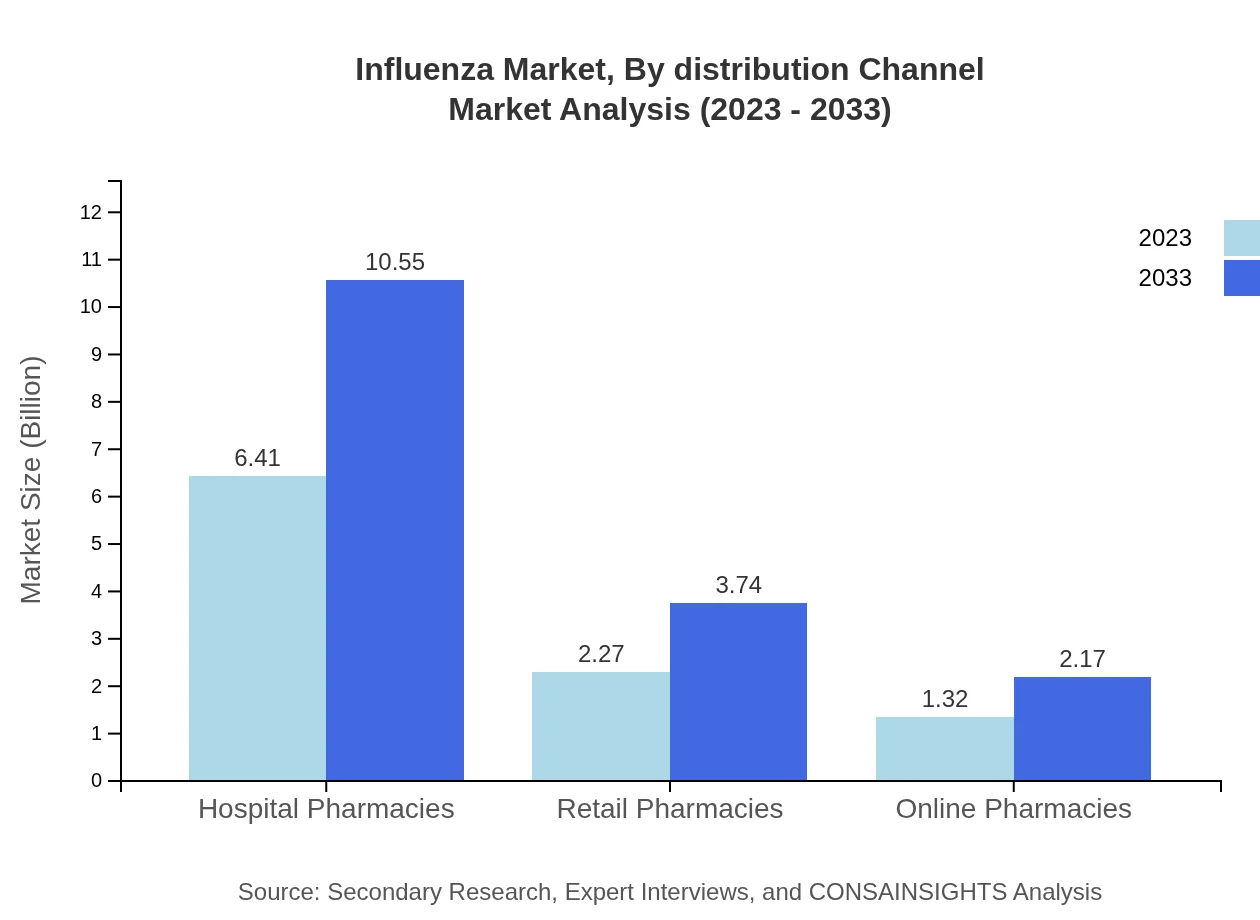
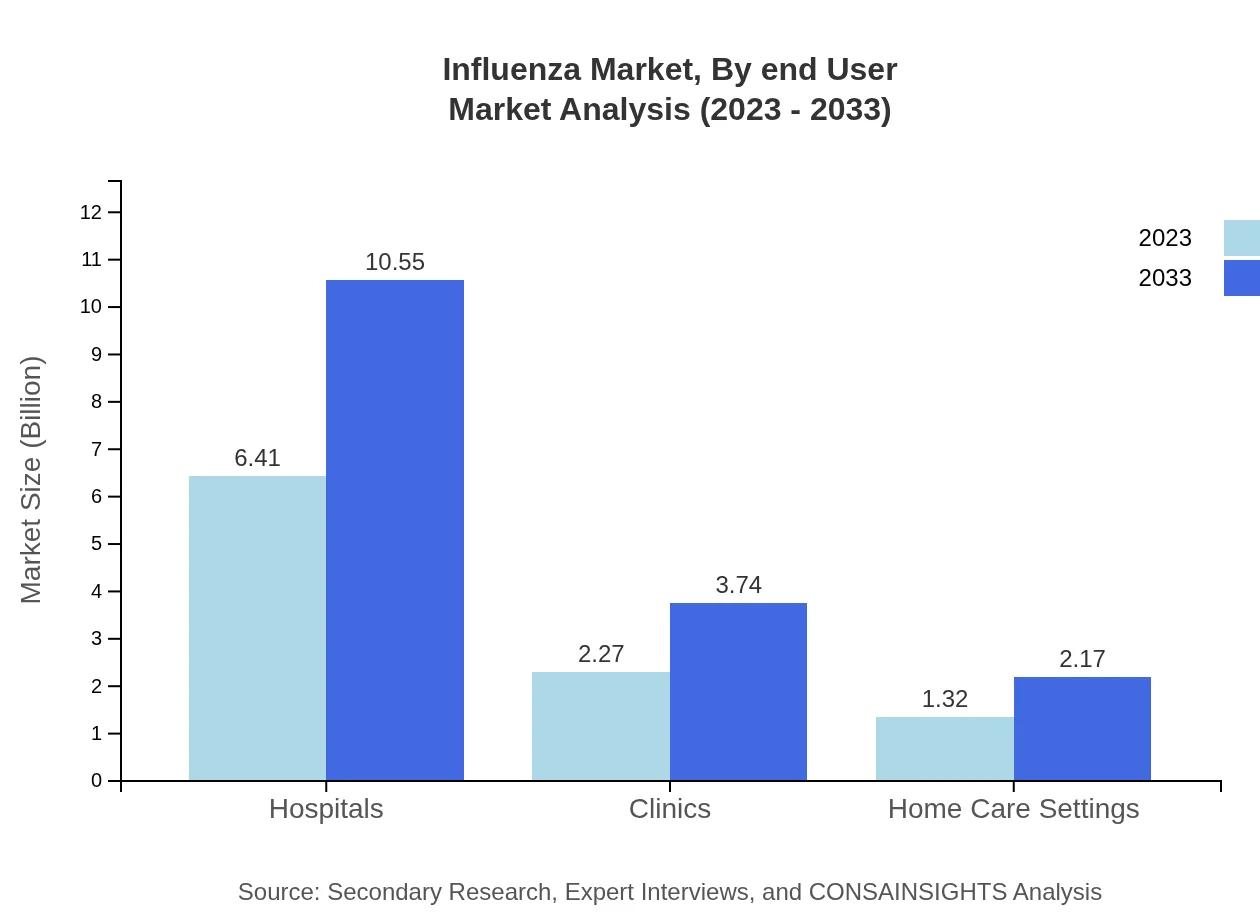
The distribution channels for influenza vaccines include Hospitals, Clinics, and Pharmacies. Hospitals dominate, with a market size of $6.41 billion in 2023, projected to rise to $10.55 billion by 2033, accounting for 64.1% of the market. Clinics provide a smaller but significant share, rising from $2.27 billion to $3.74 billion, while Home Care Settings reflect a growth from $1.32 billion to $2.17 billion.
Influenza Market Analysis By End User
The market by end-users includes individuals receiving vaccination from healthcare providers or through institutional programs. The growing focus on public health is pushing higher vaccination rates among populations, thereby increasing market size fundamentally, particularly in health-conscious populations and vulnerable groups.
Influenza Market Analysis By Region
Global Influenza Market, By Region Market Analysis (2023 - 2033)
The continental analysis demonstrates varying growth rates driven by healthcare policies, trends in disease prevalence, and immunization strategies. Regions like North America and Europe are leading due to established healthcare frameworks while Asia-Pacific shows promising growth potential due to evolving healthcare systems.
Influenza Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Influenza Industry
Sanofi Pasteur:
Sanofi Pasteur is one of the leading influenza vaccine manufacturers, known for its innovative technology in creating effective immunization products to combat seasonal and pandemic influenza.GlaxoSmithKline (GSK):
GSK is a key player in the influenza vaccine market, focusing on both seasonal and pandemic vaccines, with a strong commitment to research and development, ensuring high efficacy rates in its products.Pfizer Inc.:
Pfizer is recognized for its broad portfolio in vaccine production, including influenza vaccines, with significant investments in new research and technologies to enhance vaccine performance and availability.Novartis AG:
Novartis is a significant contributor to the influenza market, providing both seasonal and pandemic vaccines, focusing on innovation and accessibility to reach diverse populations.Merck & Co., Inc.:
Merck is an established leader in the pharmaceutical industry, with noteworthy contributions in the influenza market through the development of effective vaccines and public health initiatives.We're grateful to work with incredible clients.









FAQs
What is the market size of Influenza?
The influenza market is projected to reach a size of approximately $10 billion by 2033, growing at a compound annual growth rate (CAGR) of 5%. This growth reflects increasing awareness and demand for influenza vaccination.
What are the key market players or companies in the Influenza industry?
Key players in the influenza market include major pharmaceutical companies like GSK, Sanofi Pasteur, Seqirus, and AstraZeneca. These companies lead in vaccine manufacturing and distribution, contributing to overall market growth through innovation and strategic partnerships.
What are the primary factors driving the growth in the influenza industry?
Growth factors in the influenza market include increased vaccination campaigns, rising public awareness of influenza risks, advancements in vaccine technology, and government initiatives to promote flu vaccines as a preventive healthcare measure.
Which region is the fastest Growing in the Influenza market?
North America is the fastest-growing region in the influenza market, expected to grow from $3.40 billion in 2023 to $5.59 billion by 2033. Factors include robust healthcare infrastructure and high vaccination rates among the population.
Does ConsaInsights provide customized market report data for the Influenza industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the influenza industry. This allows for focused insights that align with strategic goals and market demands.
What deliverables can I expect from this Influenza market research project?
Deliverables from the influenza market research project include comprehensive reports containing market sizing, growth forecasts, competitive analysis, segment insights, and detailed regional breakdowns to support strategic planning.
What are the market trends of Influenza?
Key trends in the influenza market include an increasing preference for inactivated vaccines, a rise in online healthcare solutions, and a focus on seasonal vaccination programs, which together aim to enhance flu prevention strategies.