Influenza Vaccine Market Report
Published Date: 31 January 2026 | Report Code: influenza-vaccine
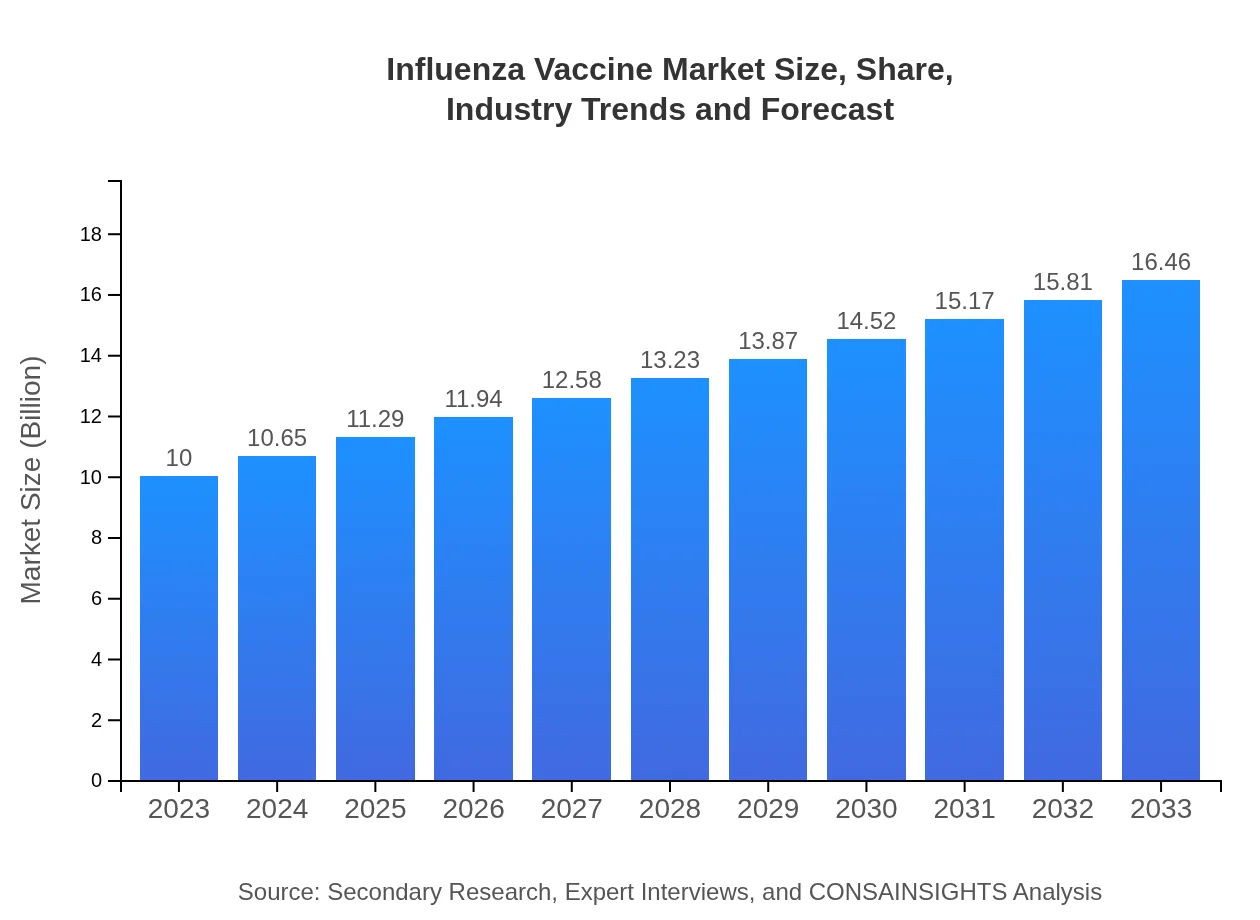
Influenza Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Influenza Vaccine market from 2023 to 2033, detailing market size, growth trends, and regional insights, alongside key market segment performances and an overview of leading players in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | Sanofi Pasteur, GlaxoSmithKline (GSK), Pfizer Inc., Novartis, Merck & Co. |
| Last Modified Date | 31 January 2026 |
Influenza Vaccine Market Overview
Customize Influenza Vaccine Market Report market research report
- ✔ Get in-depth analysis of Influenza Vaccine market size, growth, and forecasts.
- ✔ Understand Influenza Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Influenza Vaccine
What is the Market Size & CAGR of Influenza Vaccine market in 2033?
Influenza Vaccine Industry Analysis
Influenza Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Influenza Vaccine Market Analysis Report by Region
Europe Influenza Vaccine Market Report:
Europe's market for Influenza Vaccines is set to grow from $3.41 billion in 2023 to $5.61 billion by 2033, fueled by rigorous health policies and widespread vaccination programs, particularly among vulnerable populations.Asia Pacific Influenza Vaccine Market Report:
The Asia Pacific region is experiencing notable growth, with the market size projected to reach $3.12 billion by 2033, up from $1.89 billion in 2023. Increased healthcare access, rising awareness about influenza prevention, and growing vaccination programs bolster this market.North America Influenza Vaccine Market Report:
North America remains a leader in the Influenza Vaccine market with a projected market size of $5.29 billion in 2033, growing from $3.21 billion in 2023. Strong healthcare infrastructure and public awareness campaigns contribute significantly to this growth.South America Influenza Vaccine Market Report:
In South America, the market is anticipated to grow from $0.16 billion in 2023 to $0.26 billion by 2033. Although smaller in size, the expanding healthcare sector and governmental vaccination initiatives are driving demand.Middle East & Africa Influenza Vaccine Market Report:
In the Middle East and Africa, the market will increase from $1.32 billion in 2023 to $2.18 billion by 2033. Enhanced healthcare initiatives and international support for vaccination programs are essential factors driving this growth.Tell us your focus area and get a customized research report.
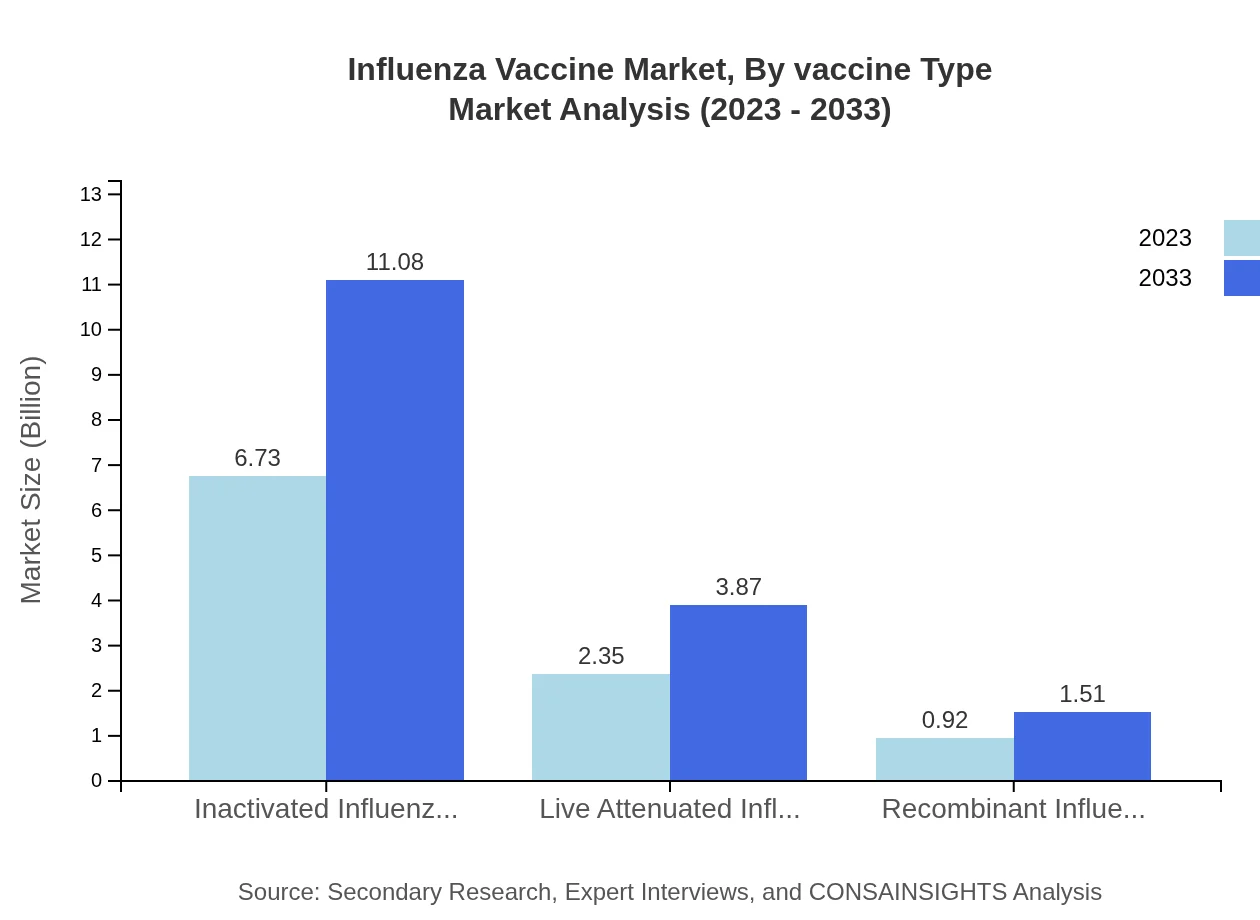
Influenza Vaccine Market Analysis By Vaccine Type
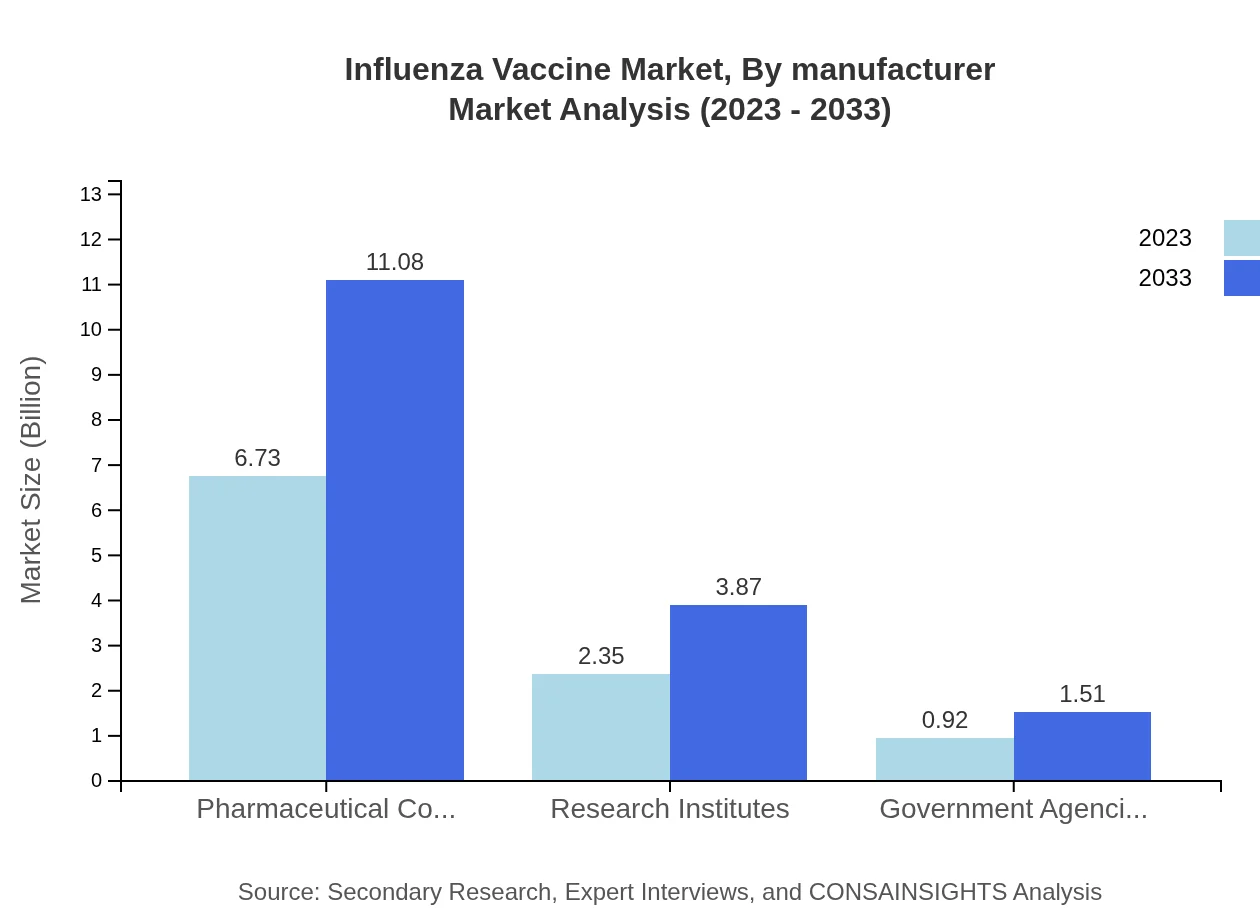
The Influenza Vaccine market, segmented by vaccine type, indicates that inactivated influenza vaccines dominate with a market size projected to grow from $6.73 billion in 2023 to $11.08 billion by 2033, accounting for 67.31% market share. Live attenuated vaccines contribute with a market growth from $2.35 billion to $3.87 billion, holding 23.53% share, while recombinant vaccines are expected to rise from $0.92 billion to $1.51 billion, maintaining 9.16% share.
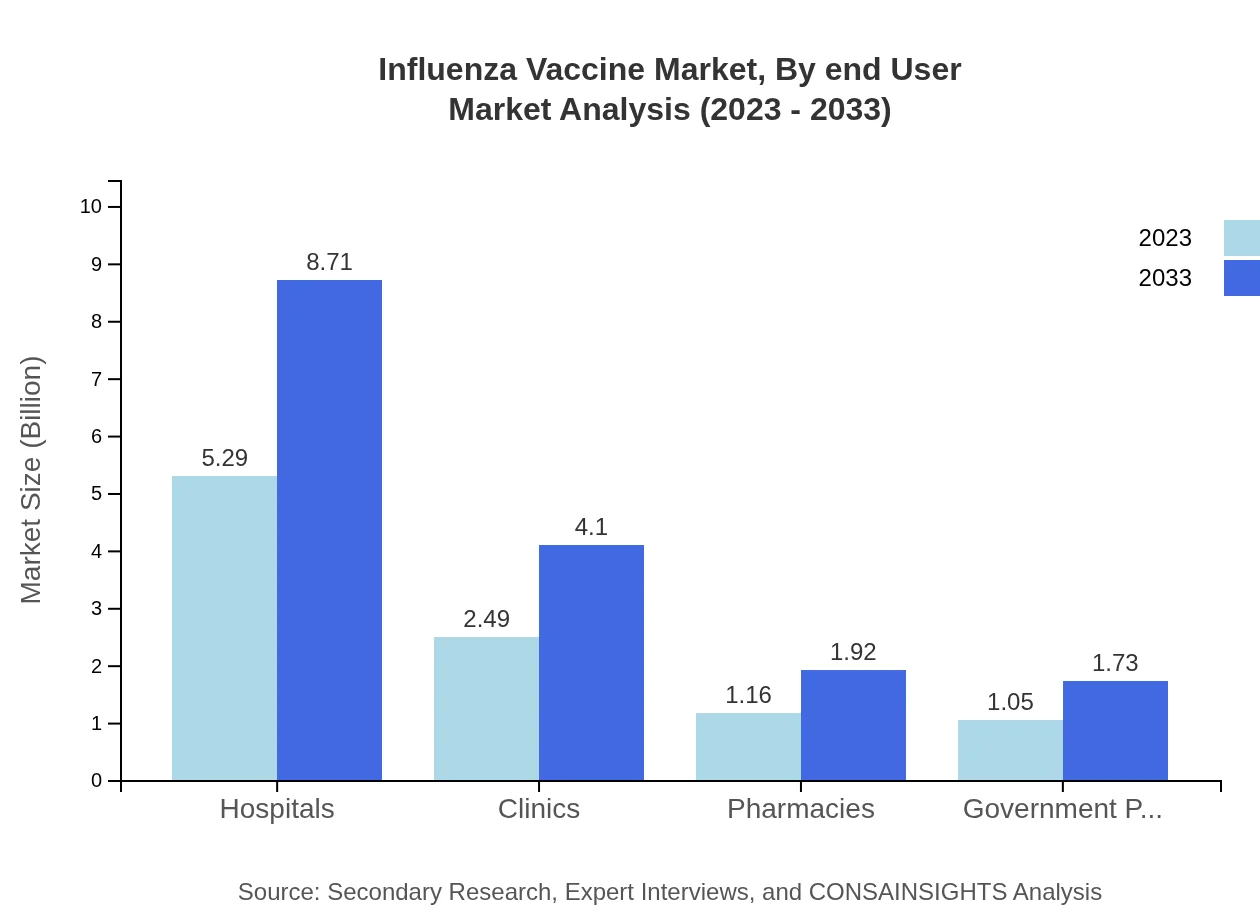
Influenza Vaccine Market Analysis By End User
In the end-user segmentation, hospitals are the leading segment growing from $5.29 billion to $8.71 billion by 2033, capturing 52.93% of the market. Clinics and pharmacies follow, expected to grow to $4.10 billion and $1.92 billion, respectively. Government programs and agencies hold a significant share, enhancing public immunization outreach, with substantial growth projected through 2033.
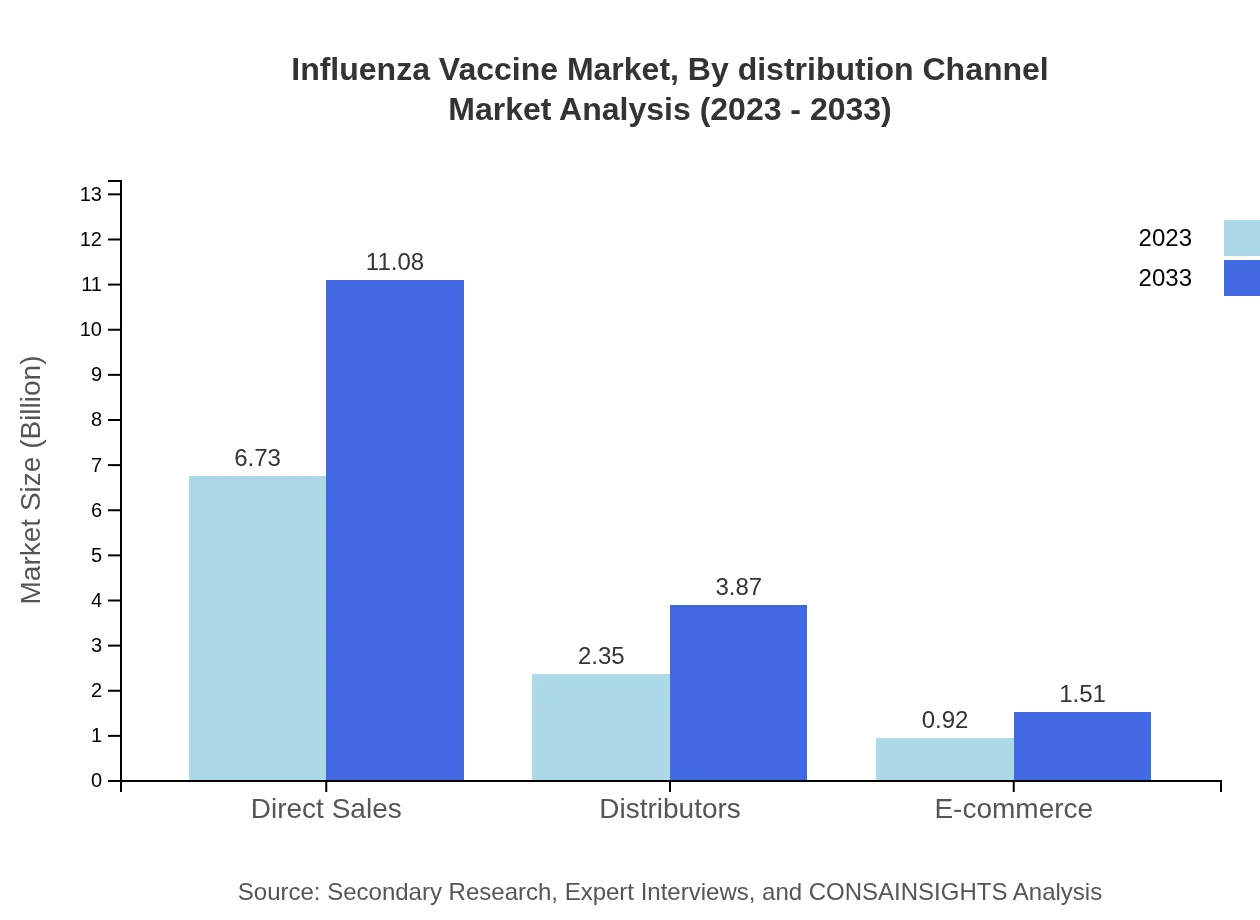
Influenza Vaccine Market Analysis By Distribution Channel
The distribution channels segment reveals critical trends, with direct sales remaining dominant, set to expand from $6.73 billion to $11.08 billion by 2033. Distributors hold a 23.53% share, with an increase to $3.87 billion, while e-commerce is evolving as a convenient channel, expected to reach $1.51 billion.
Influenza Vaccine Market Analysis By Manufacturer
Major manufacturers continue to shape the market landscape, with leading pharmaceutical companies driving innovation and reaching diverse populations through effective marketing strategies. Companies are adopting advanced manufacturing techniques to improve production efficiency and vaccine availability, crucial for meeting growing demand.
Influenza Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Influenza Vaccine Industry
Sanofi Pasteur:
A world leader in the influenza vaccine market, Sanofi Pasteur offers a range of vaccines and is heavily involved in seasonal vaccine production.GlaxoSmithKline (GSK):
GSK is known for its extensive portfolio of immunizations, including innovative influenza vaccines tied to global vaccination programs.Pfizer Inc.:
Pfizer focuses on innovative vaccine technology, bolstering its position in influenza vaccine production and sales.Novartis:
A key player in developing state-of-the-art flu vaccines, especially in Europe and North America, addressing seasonal flu challenges.Merck & Co.:
Merck engages in extensive R&D for influenza vaccines, contributing significantly to global manufacturing capabilities.We're grateful to work with incredible clients.









FAQs
What is the market size of the influenza vaccine?
The global influenza vaccine market is projected to reach a size of $10 billion by 2033, growing at a CAGR of 5%. With advancements in vaccine development and increasing awareness, the market is poised for significant growth over the forthcoming years.
What are the key market players or companies in the influenza vaccine industry?
Key players in the influenza vaccine market include major pharmaceutical companies such as Sanofi, GlaxoSmithKline, and Seqirus, which play a significant role in market supply, R&D, and distribution of various influenza vaccine types.
What are the primary factors driving the growth in the influenza vaccine industry?
Market growth is largely driven by increasing influenza incidences, heightened public awareness regarding vaccination benefits, government initiatives for vaccination drives, and continuous technological advancements in vaccine production and distribution.
Which region is the fastest Growing in the influenza vaccine market?
Europe is currently the fastest-growing region in the influenza vaccine market, projected to grow from $3.41 billion in 2023 to $5.61 billion by 2033, reflecting increasing vaccination rates and healthcare investments.
Does ConsaInsights provide customized market report data for the influenza vaccine industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the influenza vaccine industry, ensuring businesses receive relevant insights and analytics for strategic decision-making.
What deliverables can I expect from this influenza vaccine market research project?
Deliverables typically include comprehensive market analysis reports, segment-specific insights, competitor analysis, trend forecasts, and data visualizations tailored to the influenza vaccine market.
What are the market trends of the influenza vaccine?
Current market trends indicate a shift towards more effective vaccine types, including inactivated and recombinant vaccines. Additionally, the rise of e-commerce for vaccine distribution is shaping how vaccines are accessible.