Inhaled Nitric Oxide Market Report
Published Date: 31 January 2026 | Report Code: inhaled-nitric-oxide
Inhaled Nitric Oxide Market Size, Share, Industry Trends and Forecast to 2033
This market report provides a comprehensive analysis of the Inhaled Nitric Oxide industry, covering market trends, size, growth forecasts, regional insights, and leading companies from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
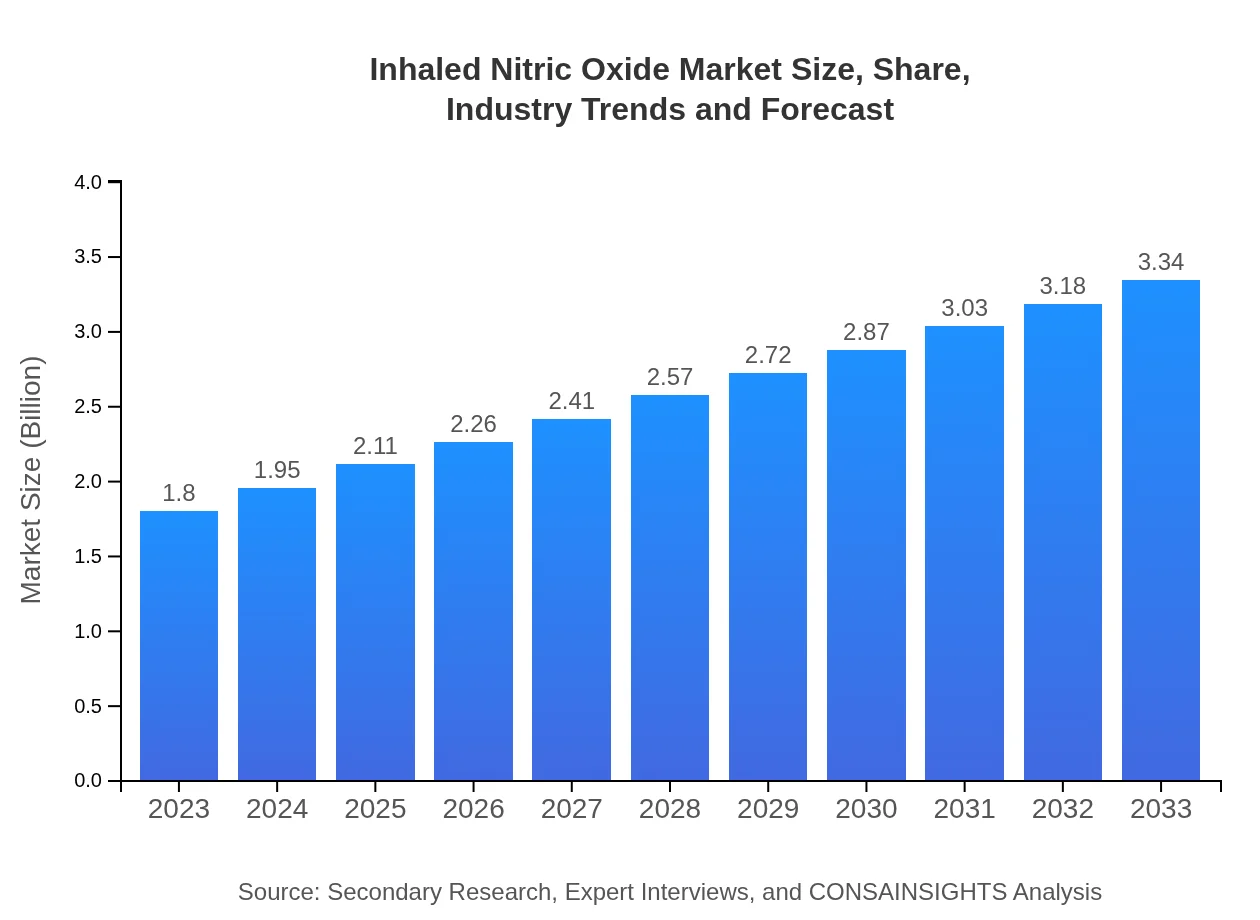
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $3.34 Billion |
| Top Companies | Mallinckrodt Pharmaceuticals, Oxidative Stress Solutions, Perrigo Company Plc, Glenmark Pharmaceuticals |
| Last Modified Date | 31 January 2026 |
Inhaled Nitric Oxide Market Overview
Customize Inhaled Nitric Oxide Market Report market research report
- ✔ Get in-depth analysis of Inhaled Nitric Oxide market size, growth, and forecasts.
- ✔ Understand Inhaled Nitric Oxide's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Inhaled Nitric Oxide
What is the Market Size & CAGR of Inhaled Nitric Oxide market in 2023?
Inhaled Nitric Oxide Industry Analysis
Inhaled Nitric Oxide Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Inhaled Nitric Oxide Market Analysis Report by Region
Europe Inhaled Nitric Oxide Market Report:
The European market is estimated to grow from 0.46 billion USD in 2023 to 0.85 billion USD by 2033. The emphasis on clinical research, coupled with leading healthcare facilities, elevates the region's standing in the market.Asia Pacific Inhaled Nitric Oxide Market Report:
In Asia Pacific, the Inhaled Nitric Oxide market is expected to grow from approximately 0.37 billion USD in 2023 to 0.69 billion USD in 2033, driven by increasing awareness of respiratory diseases and the growing healthcare infrastructure in countries like China and India.North America Inhaled Nitric Oxide Market Report:
North America dominated the Inhaled Nitric Oxide market in 2023 with a valuation of 0.68 billion USD, expected to reach 1.27 billion USD by 2033. Factors such as advanced healthcare systems and high prevalence of respiratory conditions contribute significantly to this growth.South America Inhaled Nitric Oxide Market Report:
In South America, the market is projected to rise from 0.12 billion USD in 2023 to 0.23 billion USD by 2033, supported by improving access to healthcare and investment in medical technologies.Middle East & Africa Inhaled Nitric Oxide Market Report:
In the Middle East and Africa, the Inhaled Nitric Oxide market is anticipated to expand from 0.16 billion USD in 2023 to 0.30 billion USD by 2033. Factors such as increased healthcare investments and collaborative programs to manage respiratory diseases are pivotal to this growth.Tell us your focus area and get a customized research report.
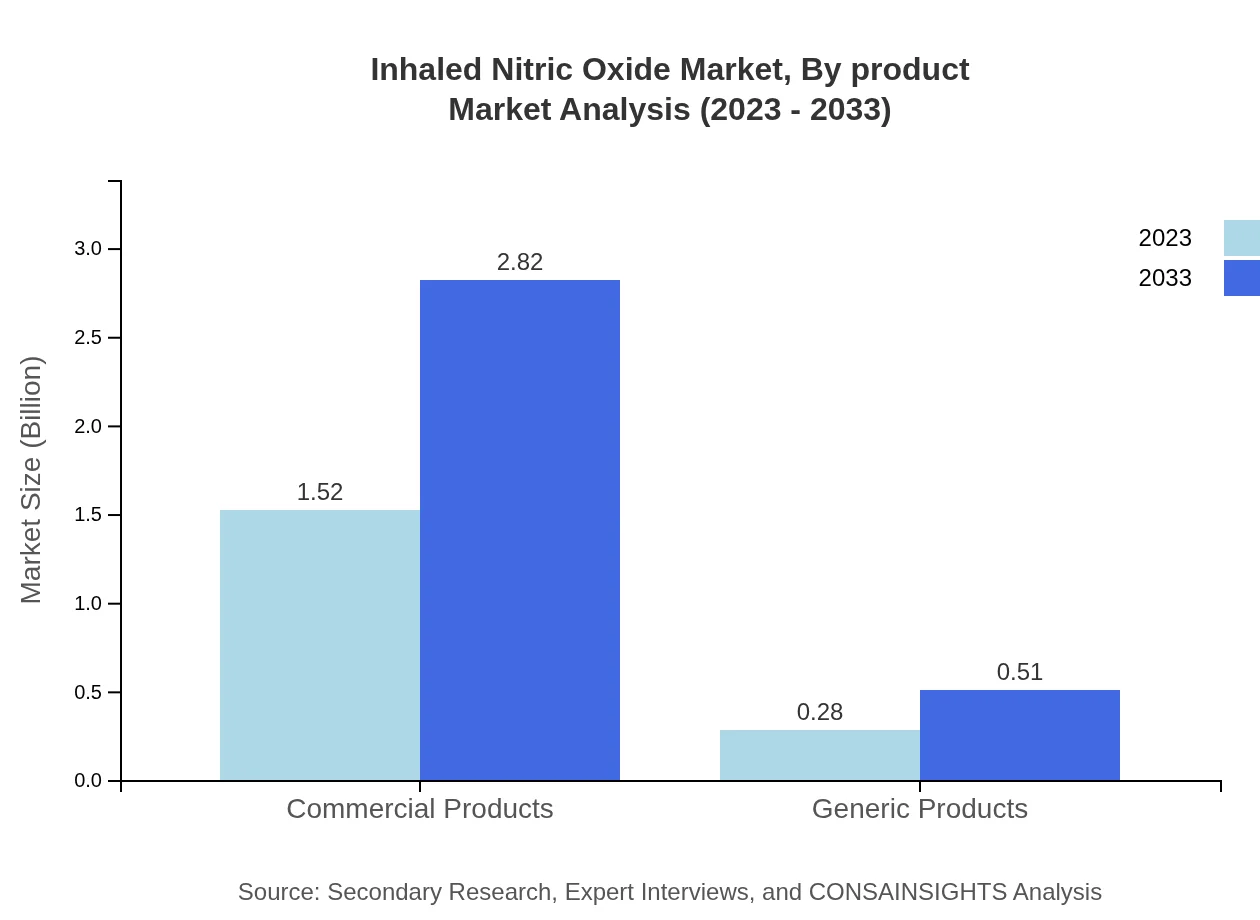
Inhaled Nitric Oxide Market Analysis By Product
The product segment consists of commercial and generic offerings, with commercial products projected to grow from 1.52 billion USD in 2023 to 2.82 billion USD by 2033, maintaining an 84.56% market share. Generic products also show promise, increasing from 0.28 billion USD in 2023 to 0.51 billion USD by 2033, capturing 15.44% of the market.
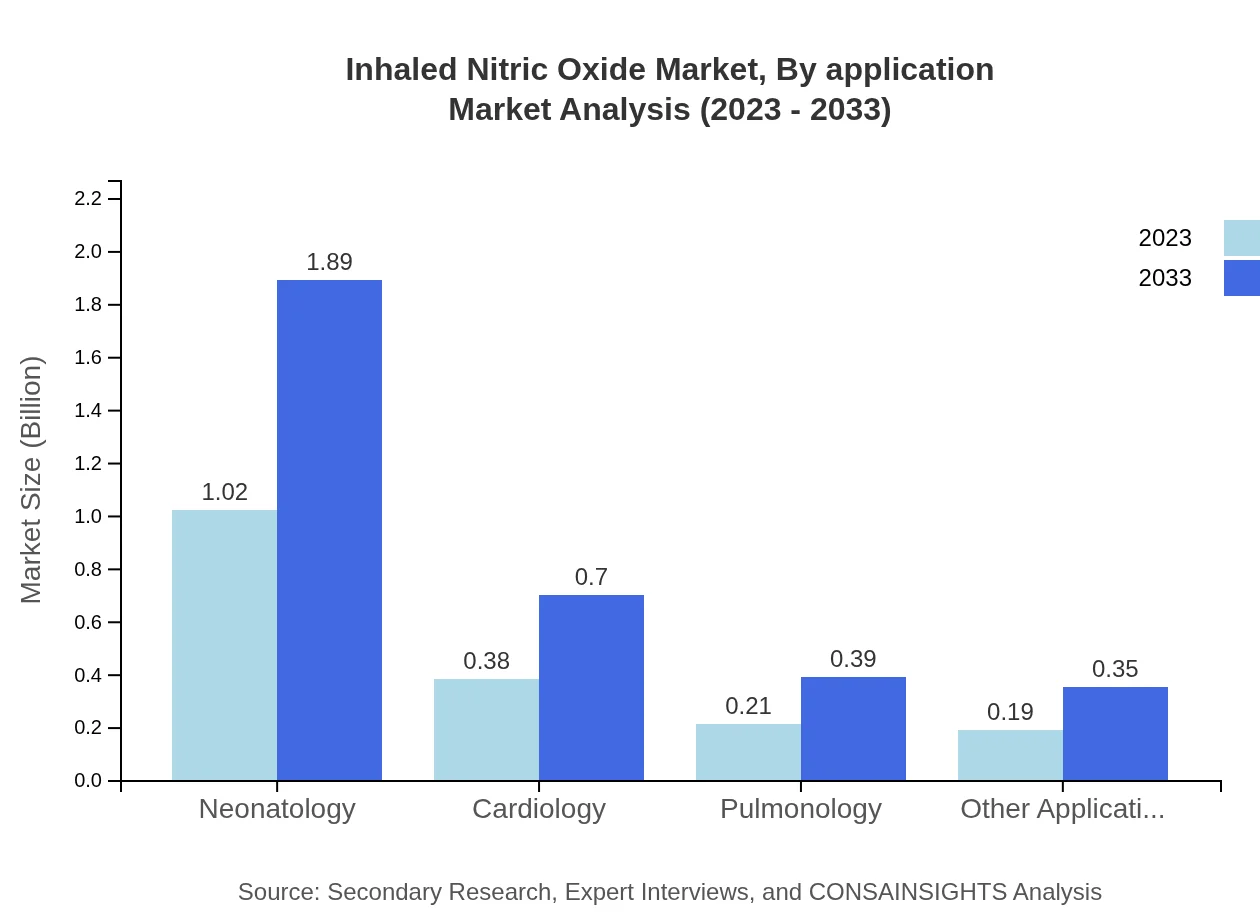
Inhaled Nitric Oxide Market Analysis By Application
Key applications include inhalation therapy, which dominates the market at 1.52 billion USD in 2023 and is expected to grow to 2.82 billion USD by 2033. Neonatology, cardiology, and pulmonology applications are also significant, reflective of the diverse therapeutic use of inhaled nitric oxide.
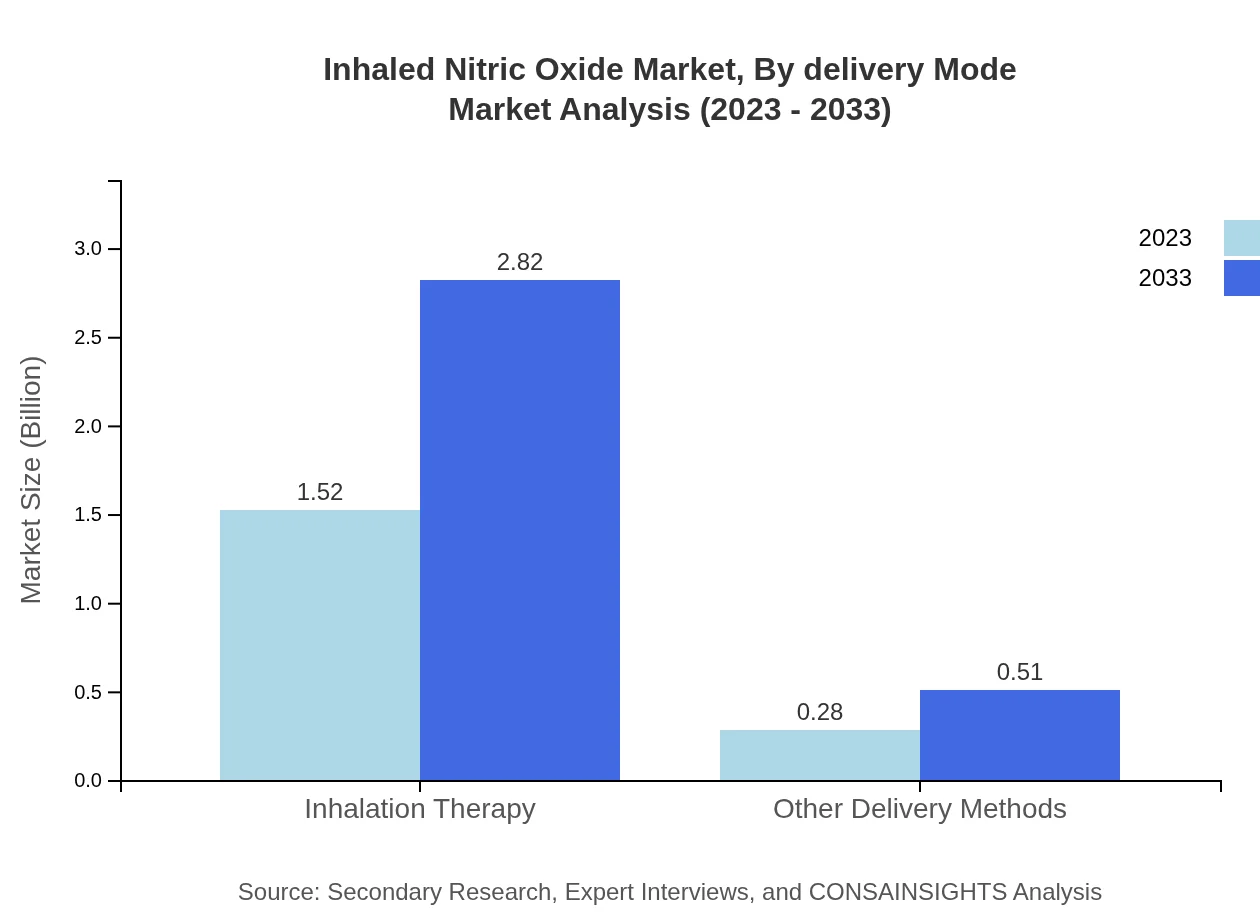
Inhaled Nitric Oxide Market Analysis By Delivery Mode
Delivery methods include direct sales, retail pharmacies, and e-commerce platforms. Direct sales are expected to dominate the market with a 65.23% share in 2023, growing to 2.18 billion USD by 2033. E-commerce and retail pharmacies also play critical roles in market penetration.
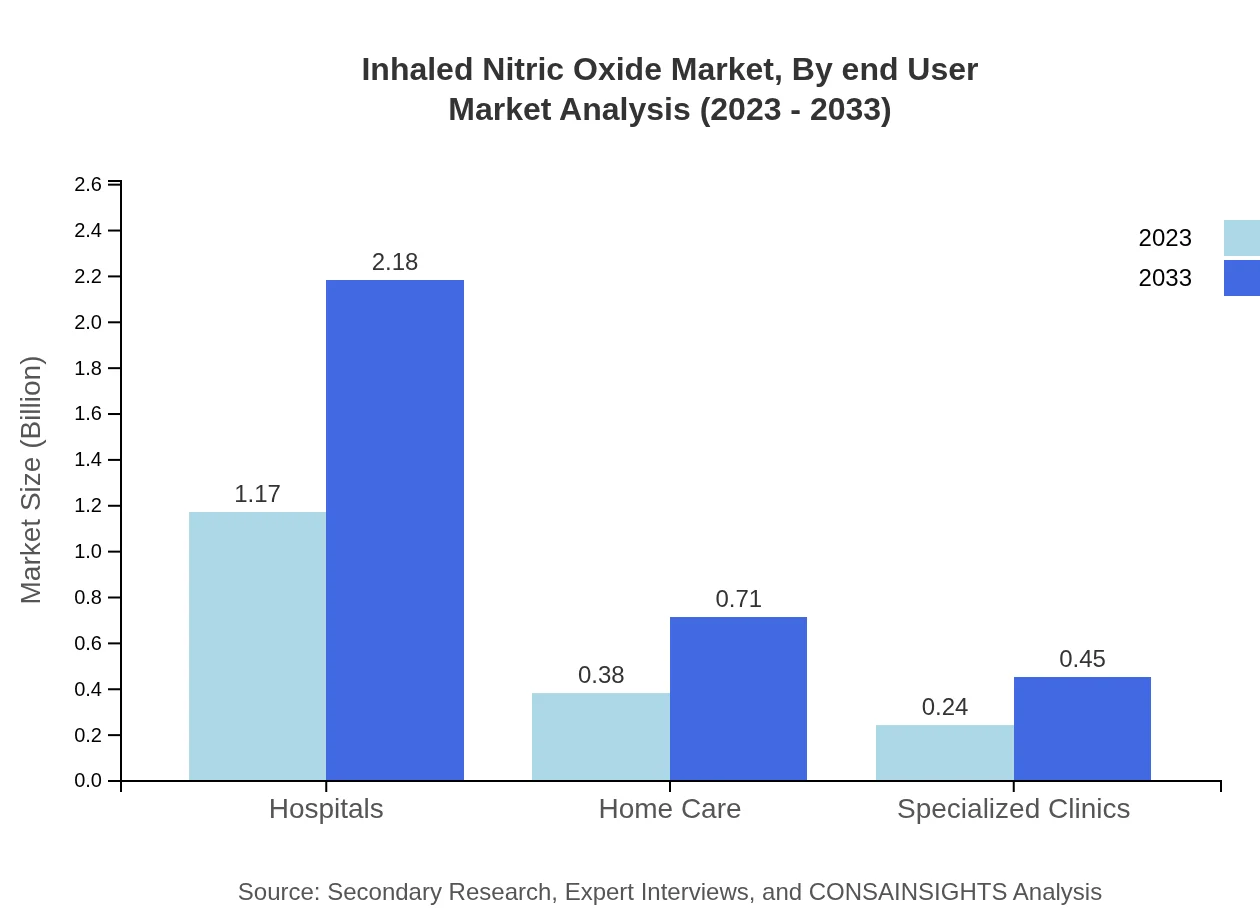
Inhaled Nitric Oxide Market Analysis By End User
End-user segmentation features hospitals, specialized clinics, and home care settings. Hospitals will retain a significant share at 65.23% in 2023, with a market growth to 2.18 billion USD projected by 2033. Other settings will also increasingly adopt inhaled nitric oxide for patient care.
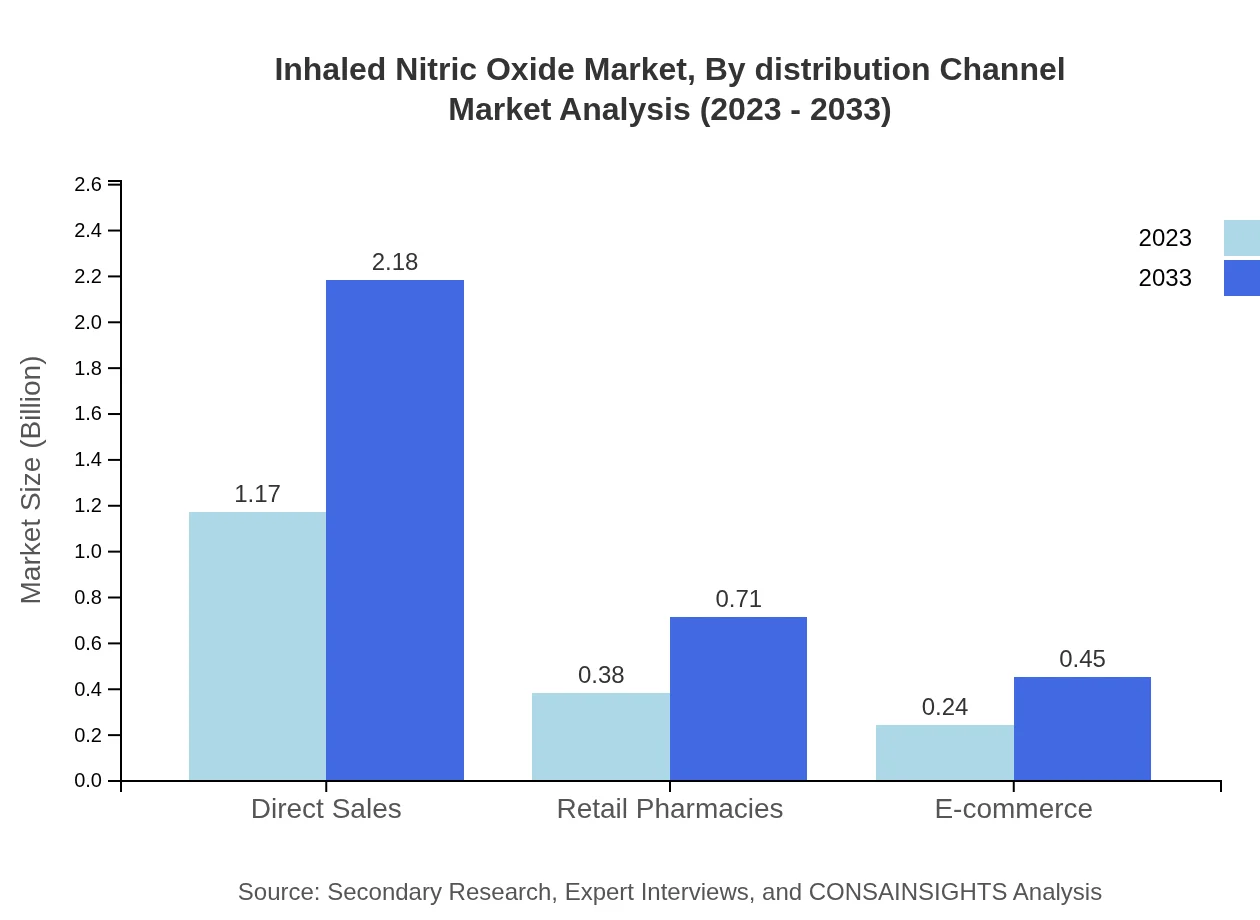
Inhaled Nitric Oxide Market Analysis By Distribution Channel
Distribution channels comprise direct sales, retail pharmacies, and e-commerce. The focus will remain on direct sales, aided by field personnel interaction with healthcare providers. Retail and online pharmacies will complement this by providing broader access to therapies.
Inhaled Nitric Oxide Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Inhaled Nitric Oxide Industry
Mallinckrodt Pharmaceuticals:
Mallinckrodt is a leading manufacturer specializing in medications for pulmonary conditions, particularly inhaled nitric oxide treatments.Oxidative Stress Solutions:
A pioneer in the development of nitric oxide therapies, focused on advancing treatment efficacy and improving patient outcomes.Perrigo Company Plc:
Perrigo is known for over-the-counter and prescription medications, contributing to the inhaled nitric oxide market through alliances and innovations.Glenmark Pharmaceuticals:
This company focuses on specialty treatments across various therapeutic areas, including respiratory health.We're grateful to work with incredible clients.









FAQs
What is the market size of inhaled Nitric Oxide?
The global inhaled nitric oxide market is projected to reach approximately $1.8 billion by 2033, growing at a CAGR of 6.2%. This growth reflects an increasing demand for nitric oxide therapies in various medical fields, including cardiology and pulmonology.
What are the key market players or companies in this inhaled Nitric Oxide industry?
Key players in the inhaled nitric oxide market include established pharmaceutical companies and specialty biotech firms. These companies are heavily investing in research and development to enhance product offerings and cater to the growing demand for nitric oxide therapies.
What are the primary factors driving the growth in the inhaled Nitric Oxide industry?
Major drivers for market growth include the rising incidence of respiratory disorders, advancements in inhalation technologies, and increasing awareness regarding the therapeutic benefits of inhaled nitric oxide in neonatal care and other medical applications.
Which region is the fastest Growing in the inhaled Nitric Oxide?
North America is anticipated to be the fastest-growing region for inhaled nitric oxide, with market growth expected to reach $1.27 billion by 2033. This growth is fueled by advanced healthcare infrastructure and high adoption rates of innovative therapies.
Does ConsaInsights provide customized market report data for the inhaled Nitric Oxide industry?
Yes, ConsaInsights offers customized market report data for the inhaled nitric oxide industry. These tailored reports meet specific client needs, providing in-depth analysis and insights into market trends, regional performance, and competitive landscapes.
What deliverables can I expect from this inhaled Nitric Oxide market research project?
From this market research project, you can expect comprehensive reports detailing market size, growth forecasts, segmentation analysis, competitive landscape insights, and regional market dynamics related to inhaled nitric oxide therapies.
What are the market trends of inhaled Nitric Oxide?
Current market trends show a shift towards the development of generic products, increased focus on home care delivery methods, and rising interest in specialized clinic applications as healthcare providers aim for more accessible treatments.