Interventional Neurology Devices Market Report
Published Date: 31 January 2026 | Report Code: interventional-neurology-devices
Interventional Neurology Devices Market Size, Share, Industry Trends and Forecast to 2033
This report presents a comprehensive analysis of the Interventional Neurology Devices market, covering key market trends, segmentation, regional insights, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
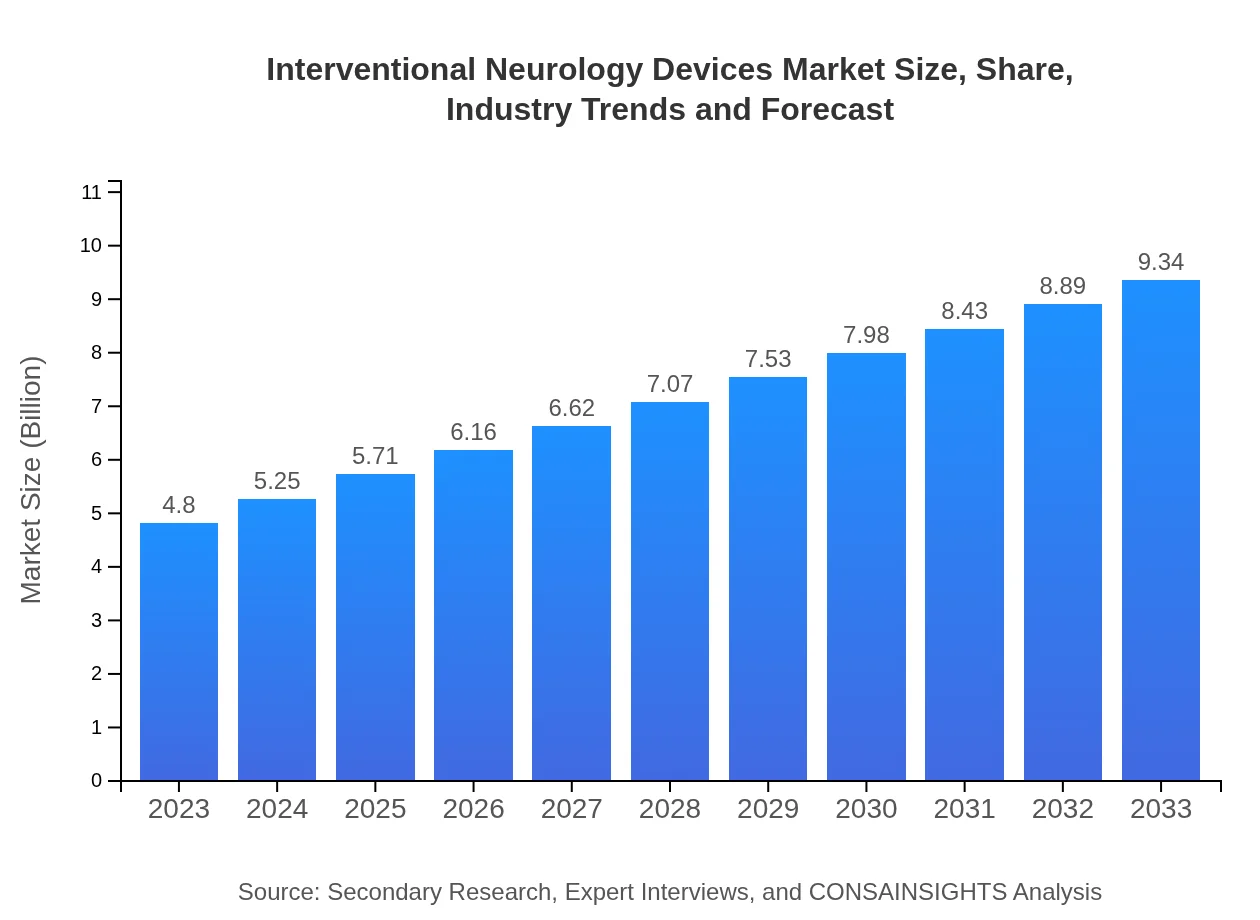
| 2023 Market Size | $4.80 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $9.34 Billion |
| Top Companies | Medtronic , Boston Scientific, Terumo Corporation, Stryker Corporation, Penumbra Inc. |
| Last Modified Date | 31 January 2026 |
Interventional Neurology Devices Market Overview
Customize Interventional Neurology Devices Market Report market research report
- ✔ Get in-depth analysis of Interventional Neurology Devices market size, growth, and forecasts.
- ✔ Understand Interventional Neurology Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Interventional Neurology Devices
What is the Market Size & CAGR of Interventional Neurology Devices market in 2023?
Interventional Neurology Devices Industry Analysis
Interventional Neurology Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Interventional Neurology Devices Market Analysis Report by Region
Europe Interventional Neurology Devices Market Report:
Europe is another critical market for Interventional Neurology Devices, with predictions indicating growth from $1.58 billion in 2023 to $3.07 billion by 2033. Factors contributing to this growth include improving patient access to innovative treatments, increasing awareness of neurovascular diseases, and supportive government policies aimed at enhancing healthcare services.Asia Pacific Interventional Neurology Devices Market Report:
The Asia Pacific region is expected to witness significant growth, with the market size projected to reach $1.85 billion by 2033, up from $0.95 billion in 2023. Factors such as the rising prevalence of neurological disorders and increased healthcare expenditure are driving market expansion. Countries like China and India are particularly contributing to this growth through improving healthcare infrastructure and policy reforms.North America Interventional Neurology Devices Market Report:
In North America, the market is poised to experience substantial growth, projected to increase from $1.55 billion in 2023 to approximately $3.01 billion by 2033. This growth is driven by advanced healthcare systems, extensive product offerings, and a strong emphasis on research and development. The United States remains a key player due to high investment in medical technologies.South America Interventional Neurology Devices Market Report:
South America presents a modest growth opportunity with the market expected to grow from $0.20 billion in 2023 to $0.40 billion by 2033. The increase can be attributed to a growing focus on enhancing healthcare services and the rising incidence of neurovascular conditions that require intervention.Middle East & Africa Interventional Neurology Devices Market Report:
The Middle East and Africa region is expected to grow from $0.52 billion in 2023 to $1.01 billion by 2033, driven by improving healthcare infrastructure and rising investments in medical technology. The growing demand for minimally invasive procedures in this region is also contributing to market growth.Tell us your focus area and get a customized research report.
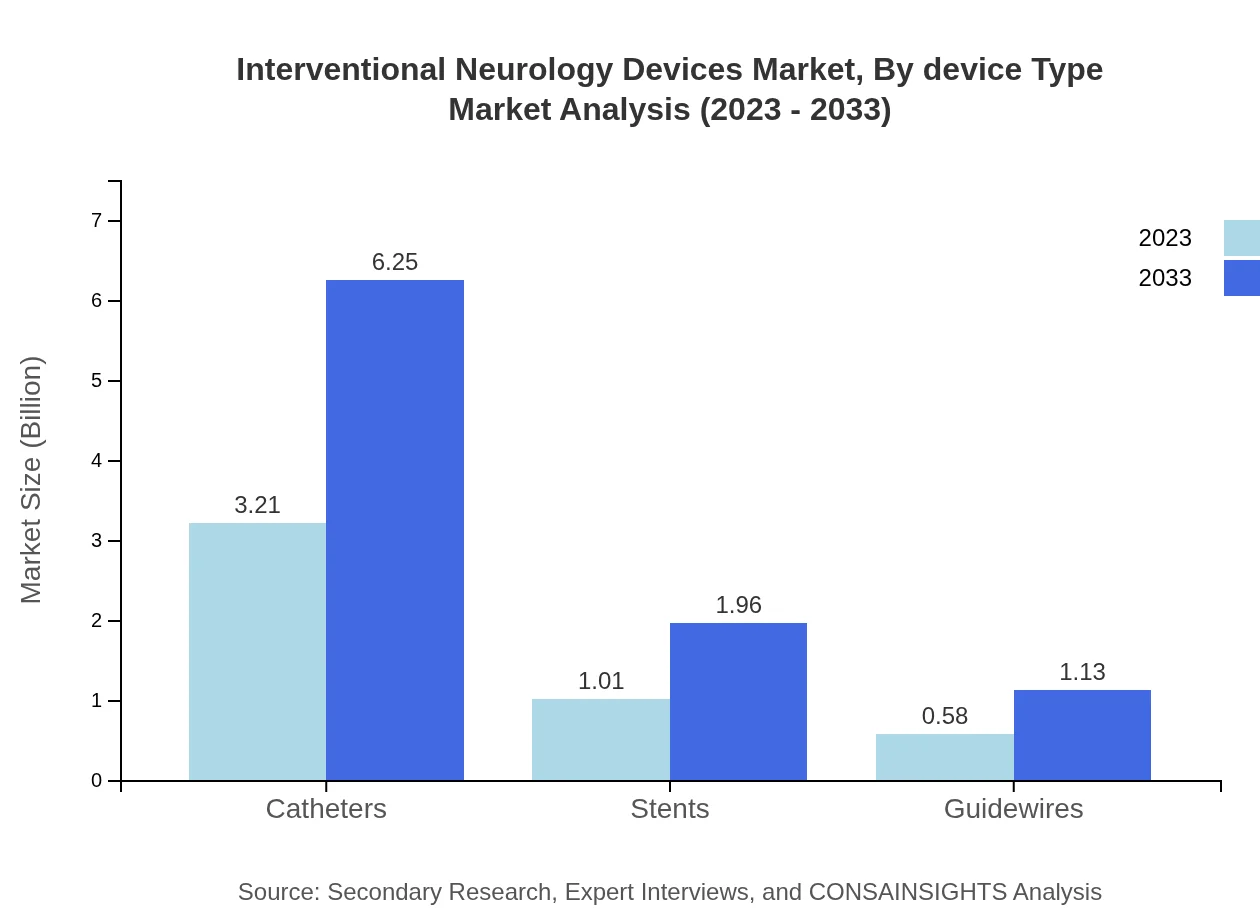
Interventional Neurology Devices Market Analysis By Device Type
The market is heavily dominated by catheters, accounting for around 66.9% market share in 2023, projected to grow to 66.9% by 2033, with sizes expanding from $3.21 billion to $6.25 billion. Other significant segments include stents and guidewires, which also play critical roles in treatment efficacy and patient outcomes.
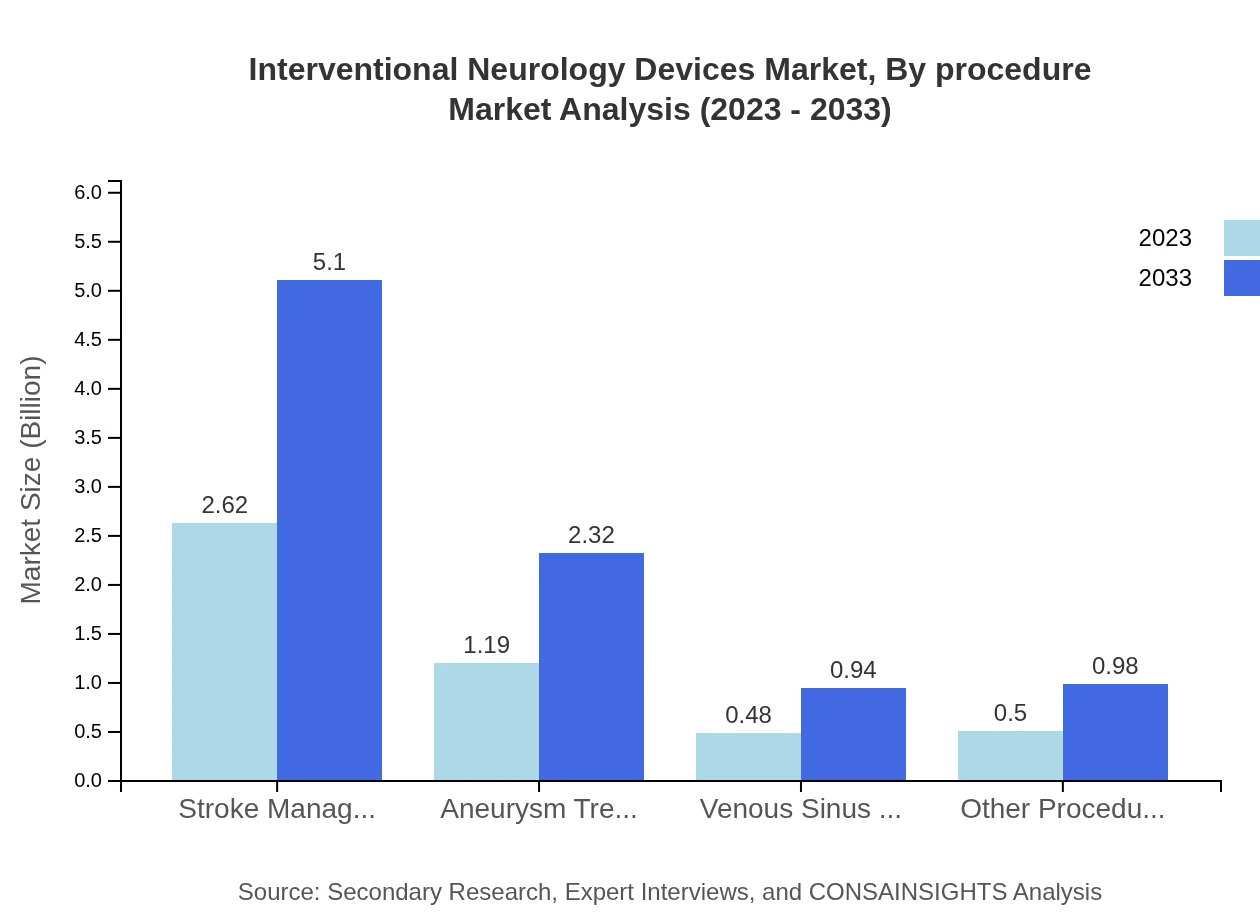
Interventional Neurology Devices Market Analysis By Procedure
The procedures segment is led by stroke management, projected to reach $5.10 billion by 2033, holding 54.58% of market share. Other noteworthy procedures include aneurysm treatment, expected to double from $1.19 billion to $2.32 billion over the same period, highlighting the need for innovative solutions to manage vascular health.
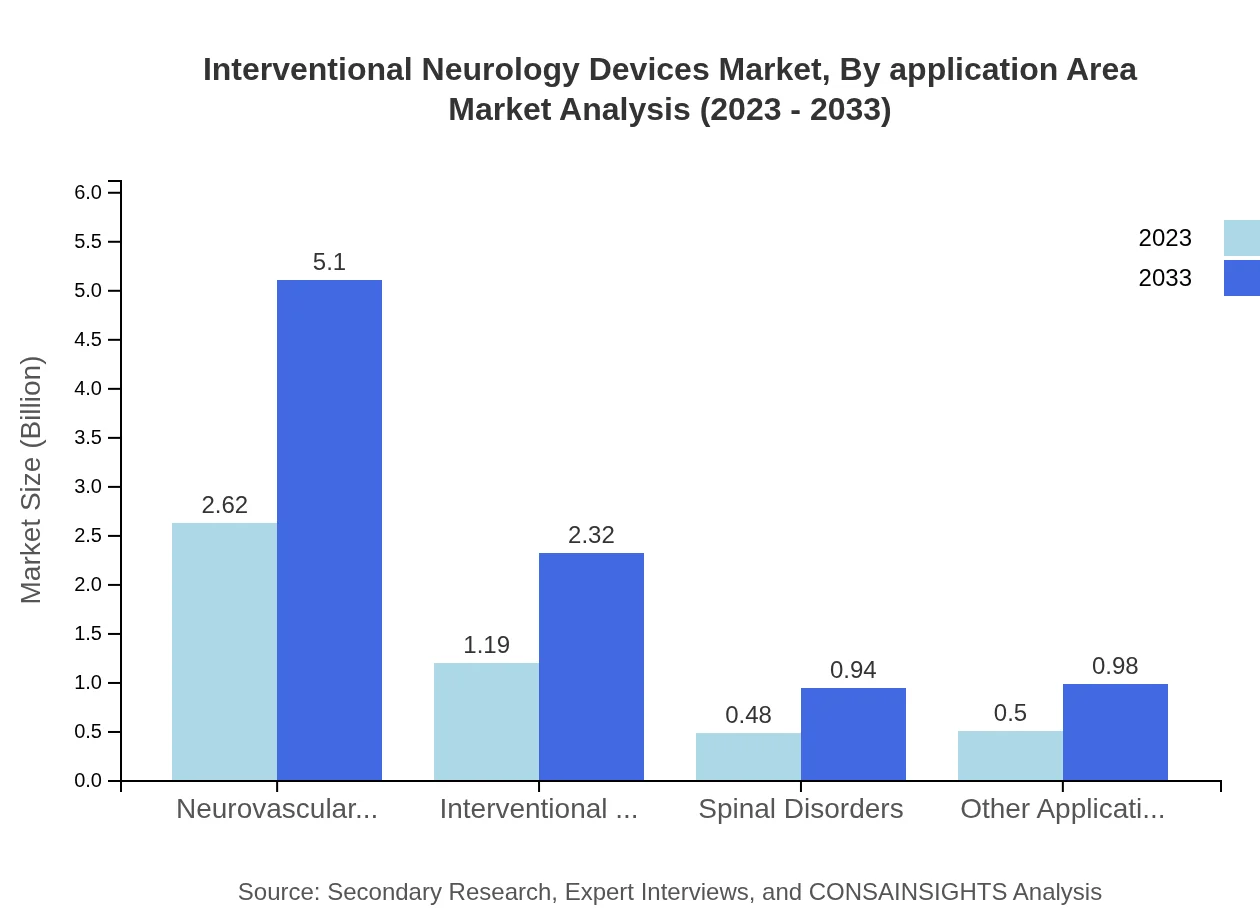
Interventional Neurology Devices Market Analysis By Application Area
The application area segment indicates a strong focus on neurovascular disorders, expected to maintain a significant market share of 54.58% and grow from $2.62 billion to $5.10 billion by 2033. This growth underscores the increasing need for specialized devices in the management of complex neurological conditions.
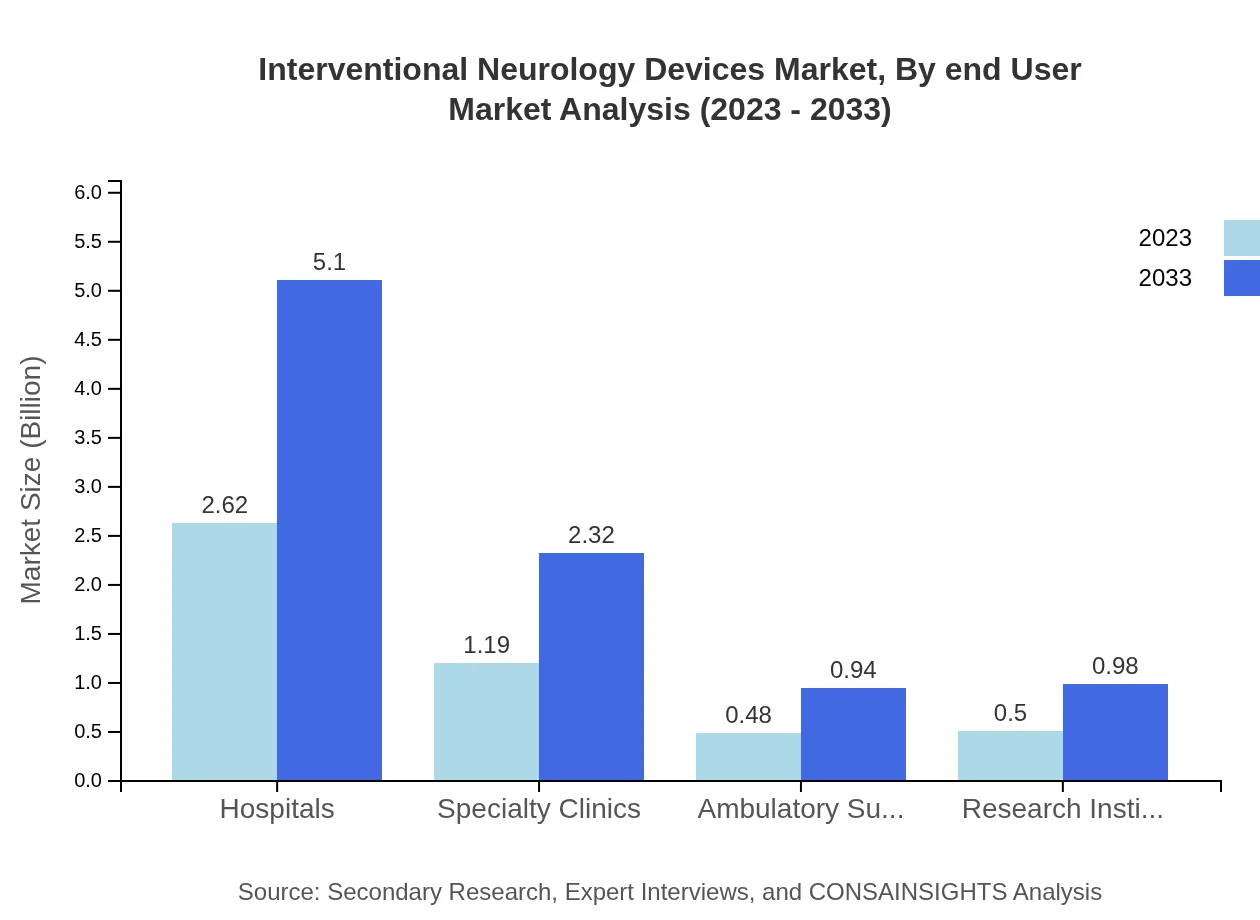
Interventional Neurology Devices Market Analysis By End User
Hospitals remain the primary end-user of Interventional Neurology Devices, holding over 54.58% share and expected to expand from $2.62 billion to $5.10 billion. Specialty clinics and ambulatory surgery centers are increasingly adopting these technologies, indicating a shift towards diversified healthcare delivery.
Interventional Neurology Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Interventional Neurology Devices Industry
Medtronic :
A global leader in medical technology and innovation, Medtronic specializes in a wide array of interventional neurology devices aimed at stroke management and aneurysm treatment.Boston Scientific:
Known for their commitment to improving patient outcomes, Boston Scientific manufactures various neurology devices and has a strong focus on minimally invasive solutions.Terumo Corporation:
Terumo specializes in minimally invasive formalities and has a notable range of devices within the interventional neurology market, focusing on innovation and patient care.Stryker Corporation:
Stryker is leadership-driven in the integration of technology in their interventional devices, providing solutions for neurovascular procedures.Penumbra Inc.:
A leader in developing innovative and minimally invasive neurovascular products, Penumbra has made significant contributions towards managing acute ischemic stroke.We're grateful to work with incredible clients.









FAQs
What is the market size of interventional Neurology Devices?
The interventional neurology devices market size is estimated at $4.8 billion in 2023, with a projected CAGR of 6.7% through 2033. This growth is indicative of the increasing demand for innovative neurological treatment solutions.
What are the key market players or companies in this interventional Neurology Devices industry?
Key players in the interventional neurology devices market include major medical device manufacturers and specialized companies committed to innovative neurovascular treatments, contributing significantly to the market's expansion through advanced technologies and strategic partnerships.
What are the primary factors driving the growth in the interventional Neurology Devices industry?
Growth in the interventional neurology devices industry is driven by an increase in neurological disorders, advancements in technology, a growing geriatric population, and improved healthcare infrastructure. These factors collectively enhance treatment efficacy and accessibility.
Which region is the fastest Growing in the interventional Neurology Devices?
Among regions, Europe represents the fastest-growing market for interventional neurology devices, with a projected increase from $1.58 billion in 2023 to $3.07 billion by 2033, fueled by rising incidences of neurological conditions and advanced healthcare initiatives.
Does ConsaInsights provide customized market report data for the interventional Neurology Devices industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the interventional neurology devices industry, ensuring that businesses receive relevant insights to support strategic decision-making.
What deliverables can I expect from this interventional Neurology Devices market research project?
Deliverables from the interventional neurology devices market research project typically include detailed market analyses, segmented data by region and application, actionable insights, trend forecasts, and strategic recommendations tailored to your business objectives.
What are the market trends of interventional Neurology Devices?
Current market trends in interventional neurology devices include increasing automation in surgical procedures, enhanced imaging technologies, a focus on minimally invasive techniques, and personalized medicine approaches, driving investment and innovation in the field.