Interventional Oncology Devices Market Report
Published Date: 31 January 2026 | Report Code: interventional-oncology-devices
Interventional Oncology Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Interventional Oncology Devices market, examining key trends, growth opportunities, and market dynamics from 2023 to 2033. The insights cover market size, segmentation, regional performance, and future forecasts, catering to industry stakeholders and decision-makers.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
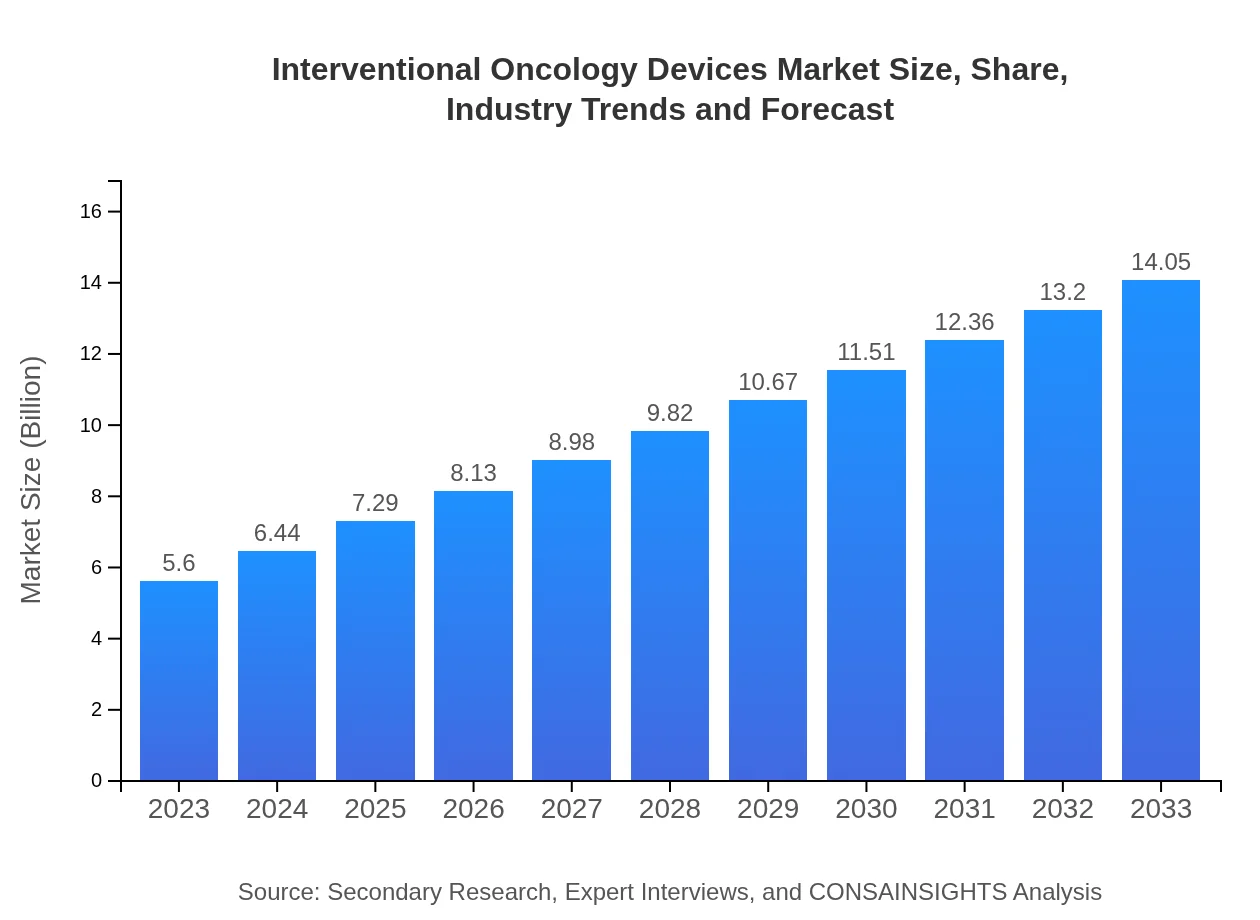
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.3% |
| 2033 Market Size | $14.05 Billion |
| Top Companies | Medtronic , Boston Scientific, AngioDynamics, Abbott Laboratories, Biogen Idec |
| Last Modified Date | 31 January 2026 |
Interventional Oncology Devices Market Overview
Customize Interventional Oncology Devices Market Report market research report
- ✔ Get in-depth analysis of Interventional Oncology Devices market size, growth, and forecasts.
- ✔ Understand Interventional Oncology Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Interventional Oncology Devices
What is the Market Size & CAGR of Interventional Oncology Devices market in 2023?
Interventional Oncology Devices Industry Analysis
Interventional Oncology Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Interventional Oncology Devices Market Analysis Report by Region
Europe Interventional Oncology Devices Market Report:
In Europe, the interventional oncology devices market is anticipated to expand from USD 1.71 billion in 2023 to USD 4.28 billion by 2033. Factors influencing this growth include increasing incidence rates of cancer, improving diagnostic capabilities, and rising demand for less invasive treatment options.Asia Pacific Interventional Oncology Devices Market Report:
In the Asia Pacific region, the interventional oncology devices market is projected to grow from USD 1.09 billion in 2023 to USD 2.74 billion by 2033. The rise is driven by increasing healthcare expenditures, growing awareness of cancer therapies, and rapid urbanization leading to improved healthcare infrastructures. Countries like Japan, China, and India are witnessing heightened demand for advanced cancer treatments.North America Interventional Oncology Devices Market Report:
North America holds a significant share in the interventional oncology devices market, forecasted to grow from USD 1.93 billion in 2023 to USD 4.85 billion by 2033. The market is driven by a high adoption rate of innovative technologies, a strong focus on research and development, and increased investment from healthcare providers in advanced cancer treatment solutions.South America Interventional Oncology Devices Market Report:
The South American market for interventional oncology devices, with a valuation of USD 0.31 billion in 2023, is expected to reach USD 0.77 billion by 2033. The market growth can be attributed to the rising prevalence of cancer, improvements in healthcare access, and government initiatives aimed at enhancing cancer care services.Middle East & Africa Interventional Oncology Devices Market Report:
For the Middle East and Africa region, the market size is poised to grow from USD 0.56 billion in 2023 to USD 1.41 billion by 2033. This growth is supported by an increase in healthcare investments and advancements in cancer awareness programs, thereby improving treatment accessibility.Tell us your focus area and get a customized research report.
Interventional Oncology Devices Market Analysis By Product
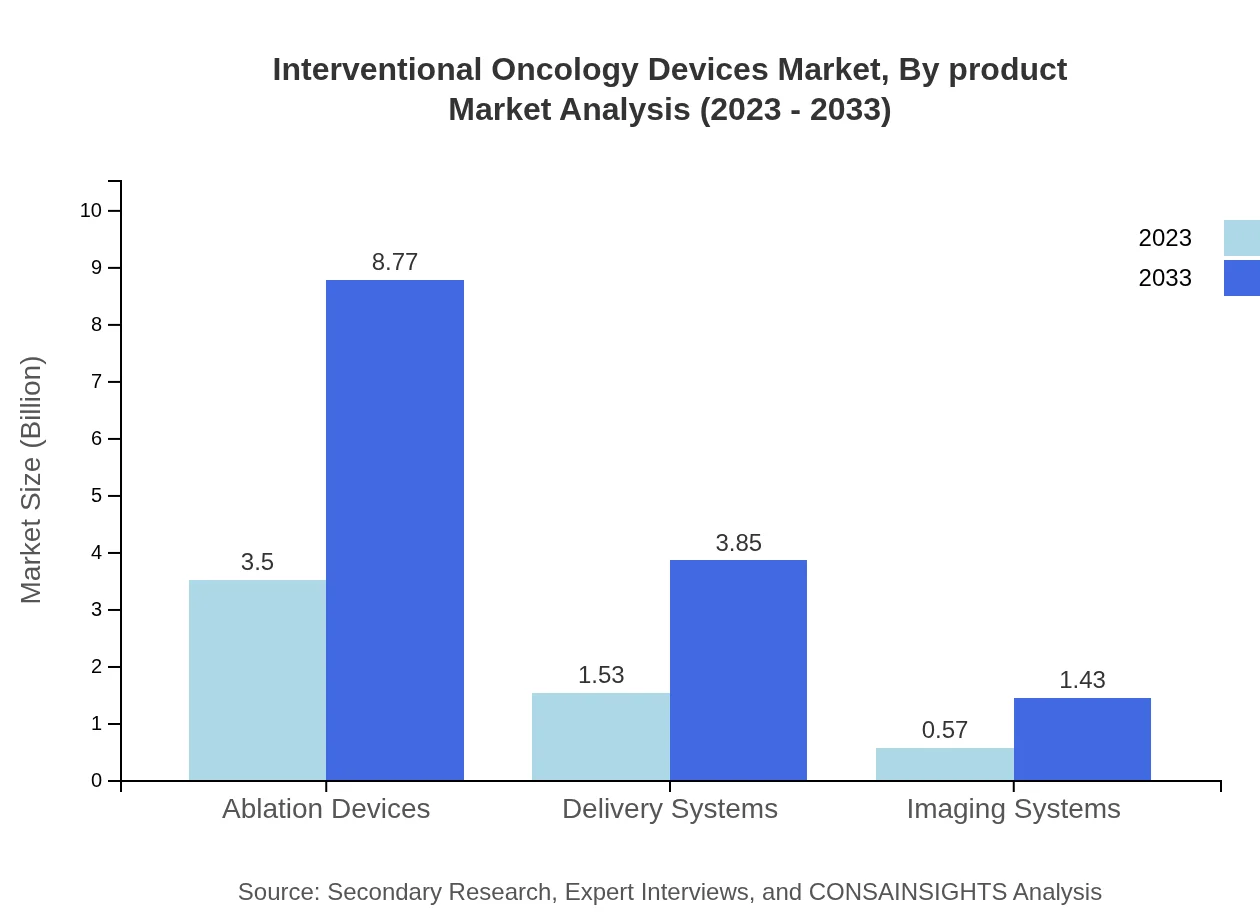
The interventional oncology devices market by product has shown significant performance across different categories. Ablation devices dominate the market share, generating USD 3.50 billion in 2023 with a projected growth to USD 8.77 billion by 2033, reflecting their critical role in cancer treatment. Delivery systems and imaging systems also play crucial roles, contributing USD 1.53 billion and USD 0.57 billion, respectively, showing increasing importance as part of integrated cancer treatment workflows.
Interventional Oncology Devices Market Analysis By Application
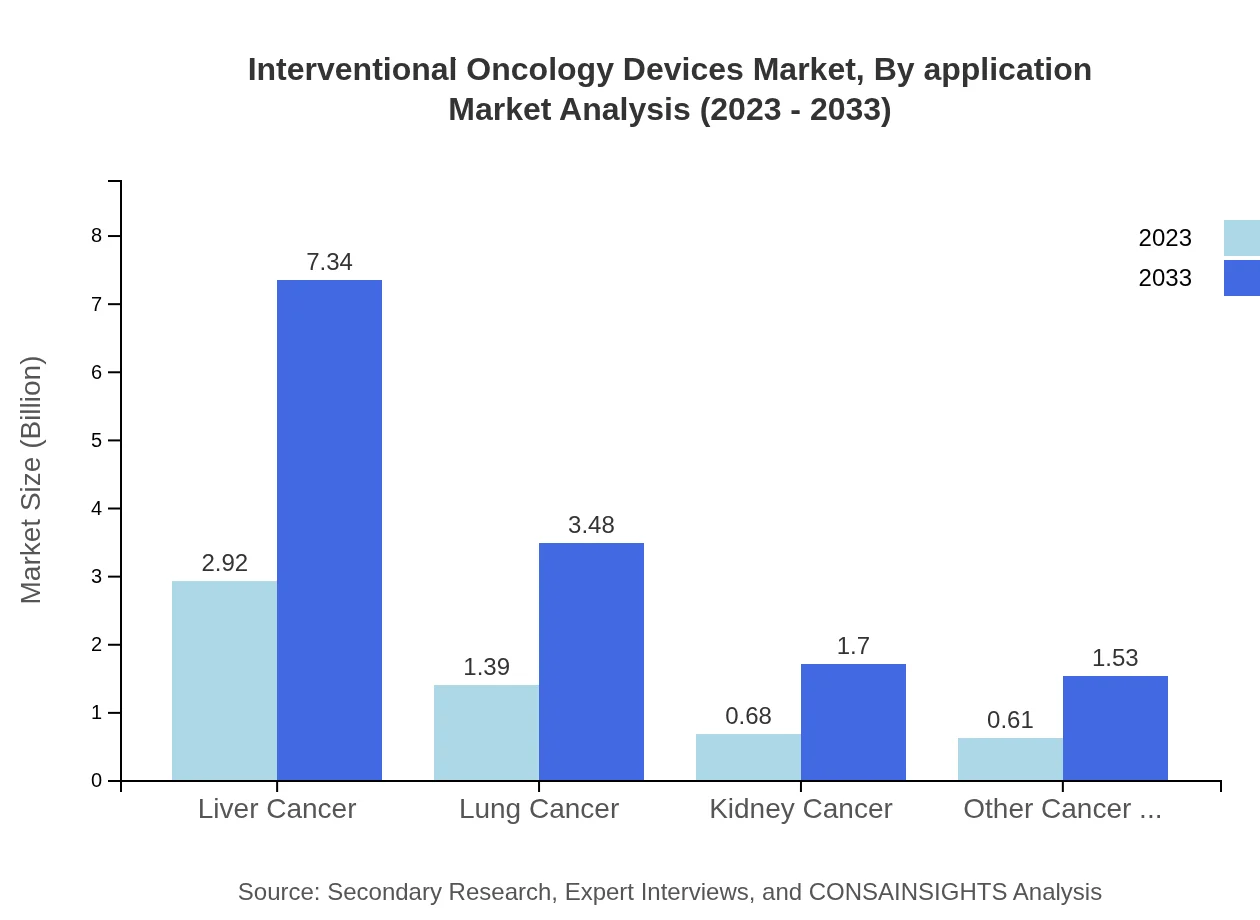
The application segmentation showcases that liver cancer treatments constitute a significant portion of the market, valued at USD 2.92 billion in 2023 and expected to reach USD 7.34 billion by 2033. Other notable segments include lung cancer and kidney cancer, which are gaining traction as treatment options become more accessible and effective.
Interventional Oncology Devices Market Analysis By End User
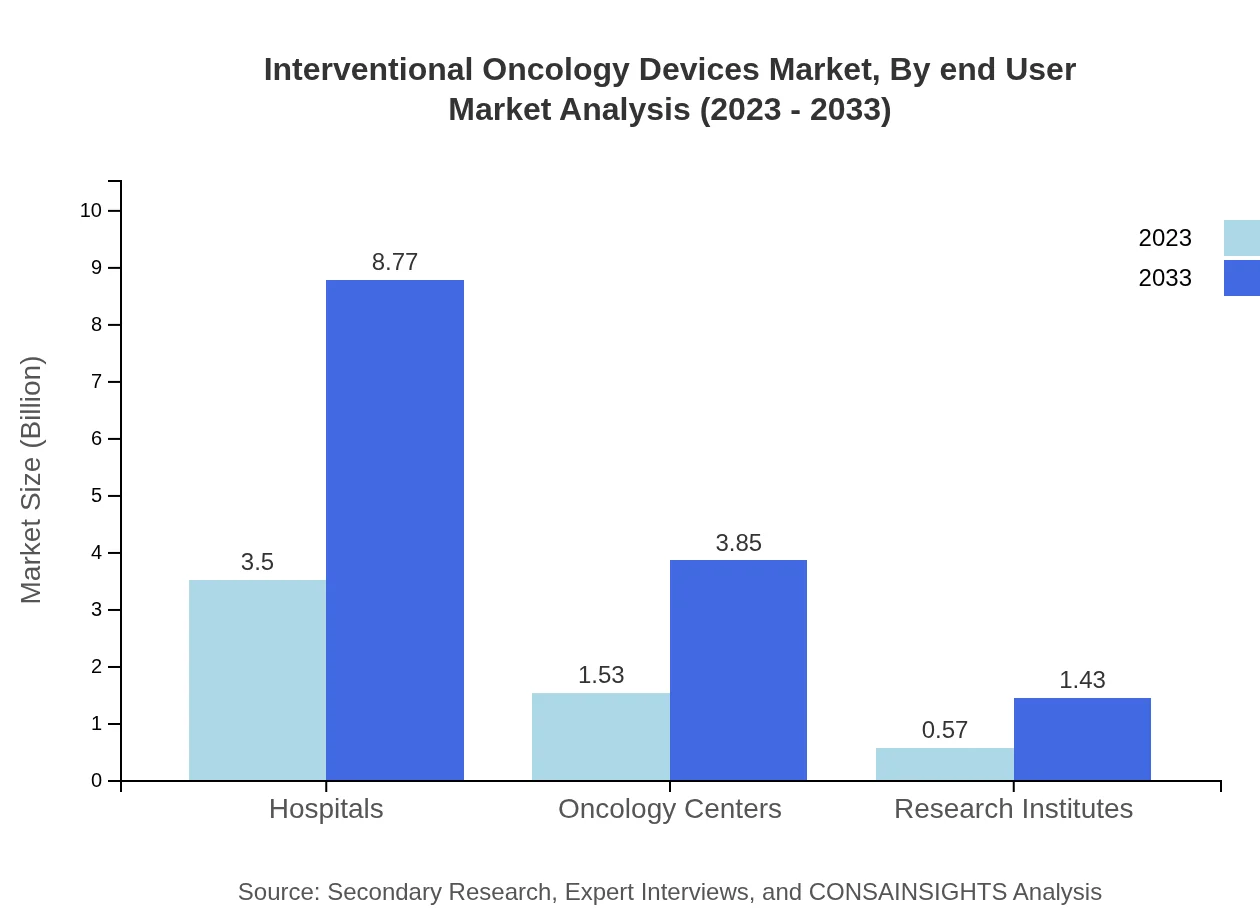
Hospitals emerge as the leading end-users in the interventional oncology devices market, with a market size of USD 3.50 billion in 2023, expected to rise to USD 8.77 billion by 2033. Oncology centers also represent a significant share, reflecting a trend towards specialized cancer treatment facilities that are equipped with advanced technology.
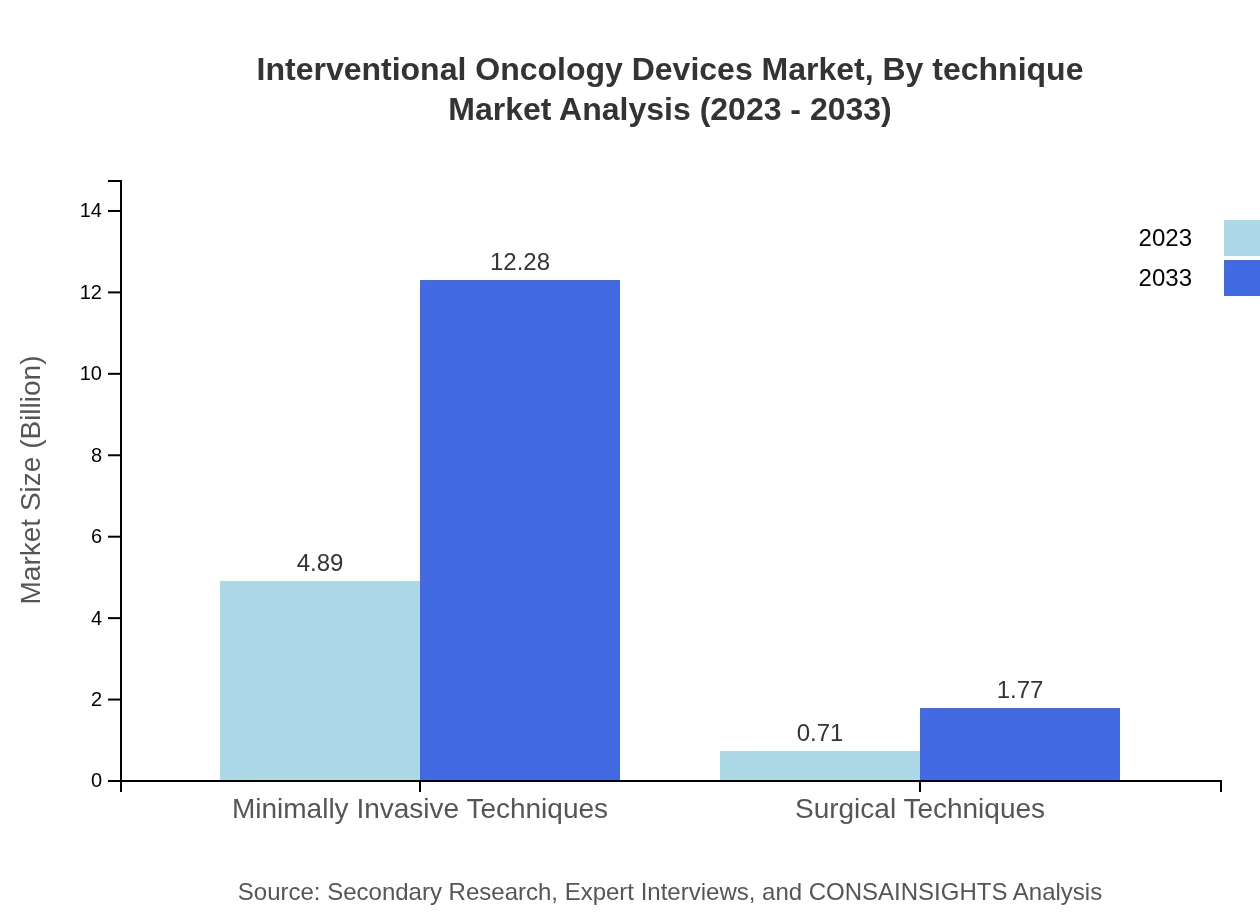
Interventional Oncology Devices Market Analysis By Technique
Minimally invasive techniques serve as the backbone of the interventional oncology devices market, with a current size of USD 4.89 billion poised for growth to USD 12.28 billion by 2033, emphasizing the preference of surgeons and patients for reduced recovery times and improved outcomes.
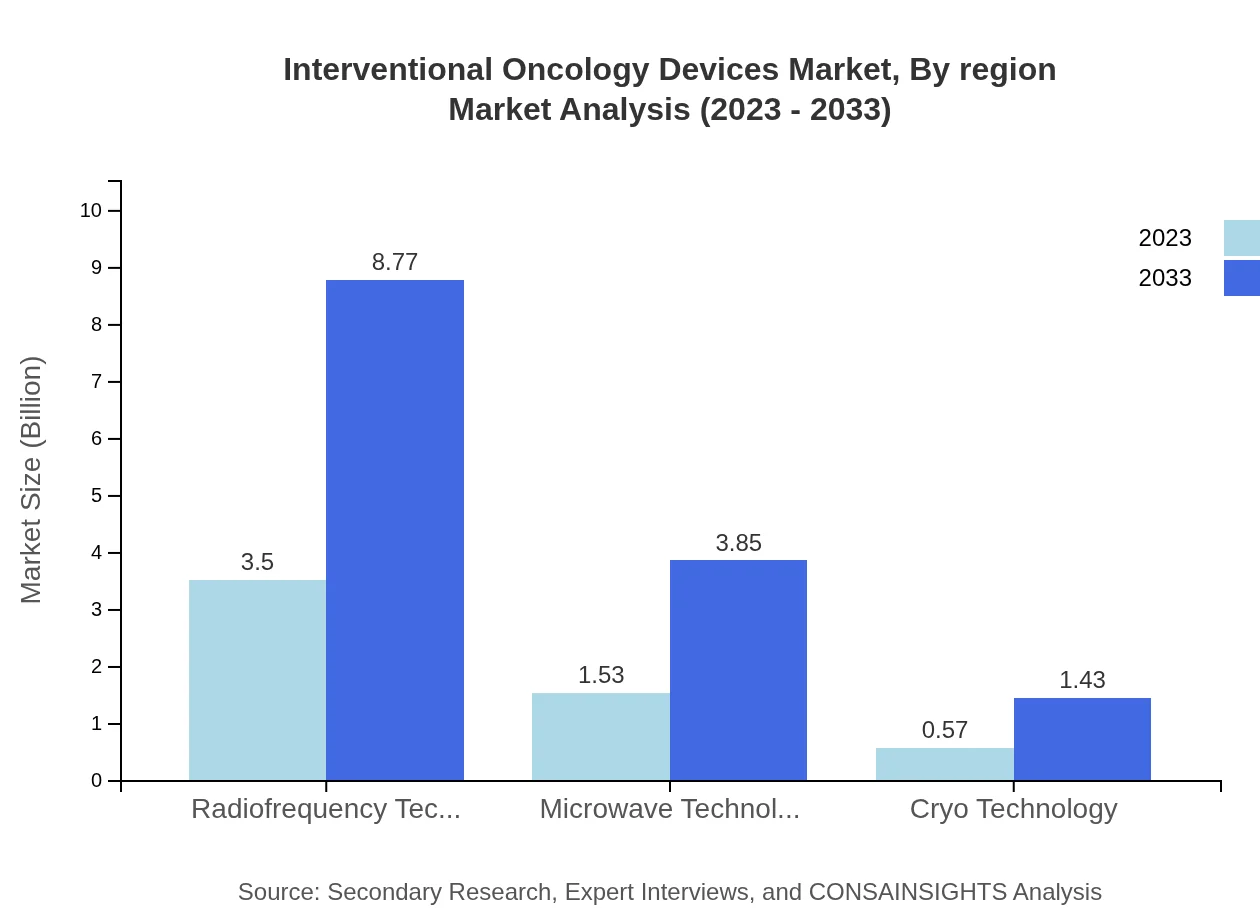
Interventional Oncology Devices Market Analysis By Region
Each technology segment, especially ablation technologies, reflects strong demand, with radiofrequency technology generating a substantial market size of USD 3.50 billion in 2023, expected to double by 2033. Emerging technologies such as microwave technology are also on the rise, showcasing growing acceptance and innovation within the treatment landscape.
Interventional Oncology Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Interventional Oncology Devices Industry
Medtronic :
Medtronic is a global leader in medical technology, providing innovative solutions for interventional oncology that cater to various cancer therapies, including advanced ablation devices.Boston Scientific:
Boston Scientific specializes in less invasive devices and technologies for cancer treatments, focusing on innovative solutions and significant contributions to procedural efficacy and patient care.AngioDynamics:
AngioDynamics is renowned for its advanced catheter-based methods and ablation technologies, pushing the envelope of precision in interventional oncology.Abbott Laboratories:
Abbott Laboratories offers a vast array of interventional devices, emphasizing research and innovation, especially in drug delivery systems integrated within oncology treatments.Biogen Idec:
Biogen Idec invests heavily in biotechnology innovations centered on cancer treatments, contributing valuable insights and products to the interventional oncology market.We're grateful to work with incredible clients.









FAQs
What is the market size of interventional Oncology Devices?
The global market size for interventional oncology devices is approximately $5.6 billion, expected to grow at a CAGR of 9.3% from 2023 to 2033. This growth reflects the increasing demand for cancer treatment technologies.
What are the key market players or companies in this interventional Oncology Devices industry?
Key players in the interventional oncology devices market include Medtronic, Boston Scientific, Abbott Laboratories, Siemens Healthineers, and AngioDynamics. These leading companies are engaged in developing innovative technologies to enhance cancer treatment efficacy.
What are the primary factors driving the growth in the interventional Oncology Devices industry?
The growth in the interventional oncology devices industry is primarily driven by the increasing prevalence of cancer, advancements in minimally invasive techniques, rising healthcare expenditure, and growing awareness of interventional oncology benefits among medical professionals and patients.
Which region is the fastest Growing in the interventional Oncology Devices?
The fastest-growing region in the interventional oncology devices market is Europe, with a market size projected to grow from $1.71 billion in 2023 to $4.28 billion by 2033. This growth is fueled by advanced healthcare infrastructure and rising cancer incidence.
Does ConsaInsights provide customized market report data for the interventional Oncology Devices industry?
Yes, ConsaInsights offers customized market report data tailored specifically for the interventional oncology devices industry. Clients can request specialized insights based on their unique requirements and market interests.
What deliverables can I expect from this interventional Oncology Devices market research project?
From the interventional oncology devices market research project, clients can expect comprehensive deliverables including detailed market analysis, growth forecasts, competitive landscape insights, and segment-specific trends, along with strategic recommendations.
What are the market trends of interventional Oncology Devices?
Current trends in the interventional oncology devices market include an increased focus on minimally invasive approaches, technological advancements in imaging systems, significant investments in R&D, and a growing emphasis on personalized medicine and targeted therapies.