Intra Abdominal Pressure Measurement Devices Market Report
Published Date: 31 January 2026 | Report Code: intra-abdominal-pressure-measurement-devices
Intra Abdominal Pressure Measurement Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive overview of the intra-abdominal pressure measurement devices market, detailing market trends, size, segmentation, regional insights, and forecasts for the period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
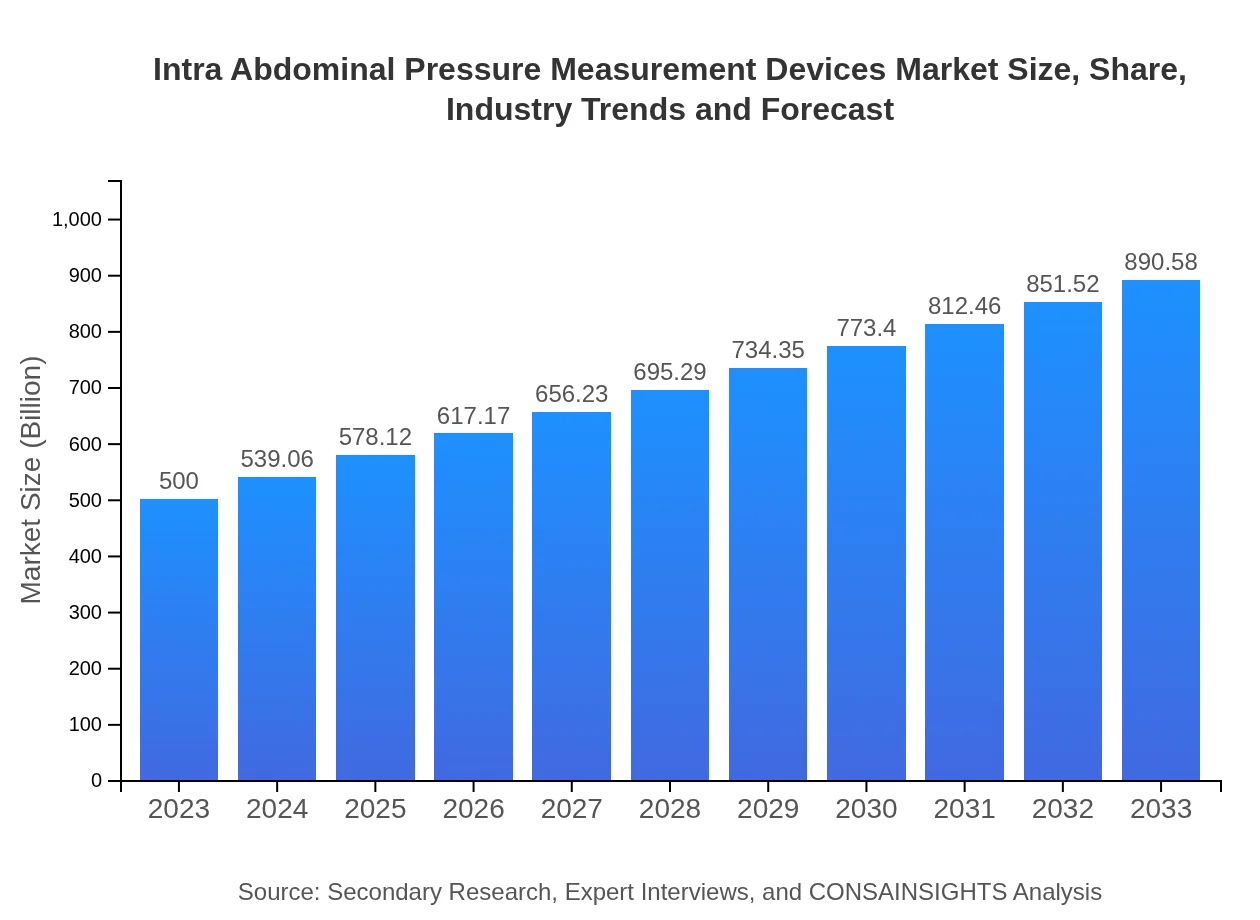
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $890.58 Million |
| Top Companies | Medtronic , Smiths Medical, C.R. Bard, a BD company, Abbott Laboratories |
| Last Modified Date | 31 January 2026 |
Intra Abdominal Pressure Measurement Devices Market Overview
Customize Intra Abdominal Pressure Measurement Devices Market Report market research report
- ✔ Get in-depth analysis of Intra Abdominal Pressure Measurement Devices market size, growth, and forecasts.
- ✔ Understand Intra Abdominal Pressure Measurement Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Intra Abdominal Pressure Measurement Devices
What is the Market Size & CAGR of Intra Abdominal Pressure Measurement Devices market in 2023?
Intra Abdominal Pressure Measurement Devices Industry Analysis
Intra Abdominal Pressure Measurement Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Intra Abdominal Pressure Measurement Devices Market Analysis Report by Region
Europe Intra Abdominal Pressure Measurement Devices Market Report:
The European market is projected to grow from $167.30 million in 2023 to $297.99 million by 2033, supported by regulatory advancements, increased awareness, and rising surgical procedures demanding intra-abdominal pressure monitoring.Asia Pacific Intra Abdominal Pressure Measurement Devices Market Report:
The Asia Pacific region is experiencing significant growth, projected to reach approximately $162.80 million by 2033 from $91.40 million in 2023. The increase is fueled by rising healthcare expenditures, urbanization, and a growing prevalence of obesity-related complications necessitating intra-abdominal monitoring.North America Intra Abdominal Pressure Measurement Devices Market Report:
North America holds the largest market share, estimated to grow from $174.25 million in 2023 to $310.37 million by 2033. This growth is attributed to advanced healthcare infrastructure, high healthcare spending, and the presence of key market players offering innovative products.South America Intra Abdominal Pressure Measurement Devices Market Report:
In South America, the market is anticipated to grow from $22.60 million in 2023 to $40.25 million by 2033, driven by improvements in healthcare systems and rising investments in medical technology innovation.Middle East & Africa Intra Abdominal Pressure Measurement Devices Market Report:
The Middle East and Africa region is expected to see growth from $44.45 million in 2023 to $79.17 million by 2033, with increasing healthcare initiatives and investments supporting market expansion.Tell us your focus area and get a customized research report.
Intra Abdominal Pressure Measurement Devices Market Analysis By Device Type
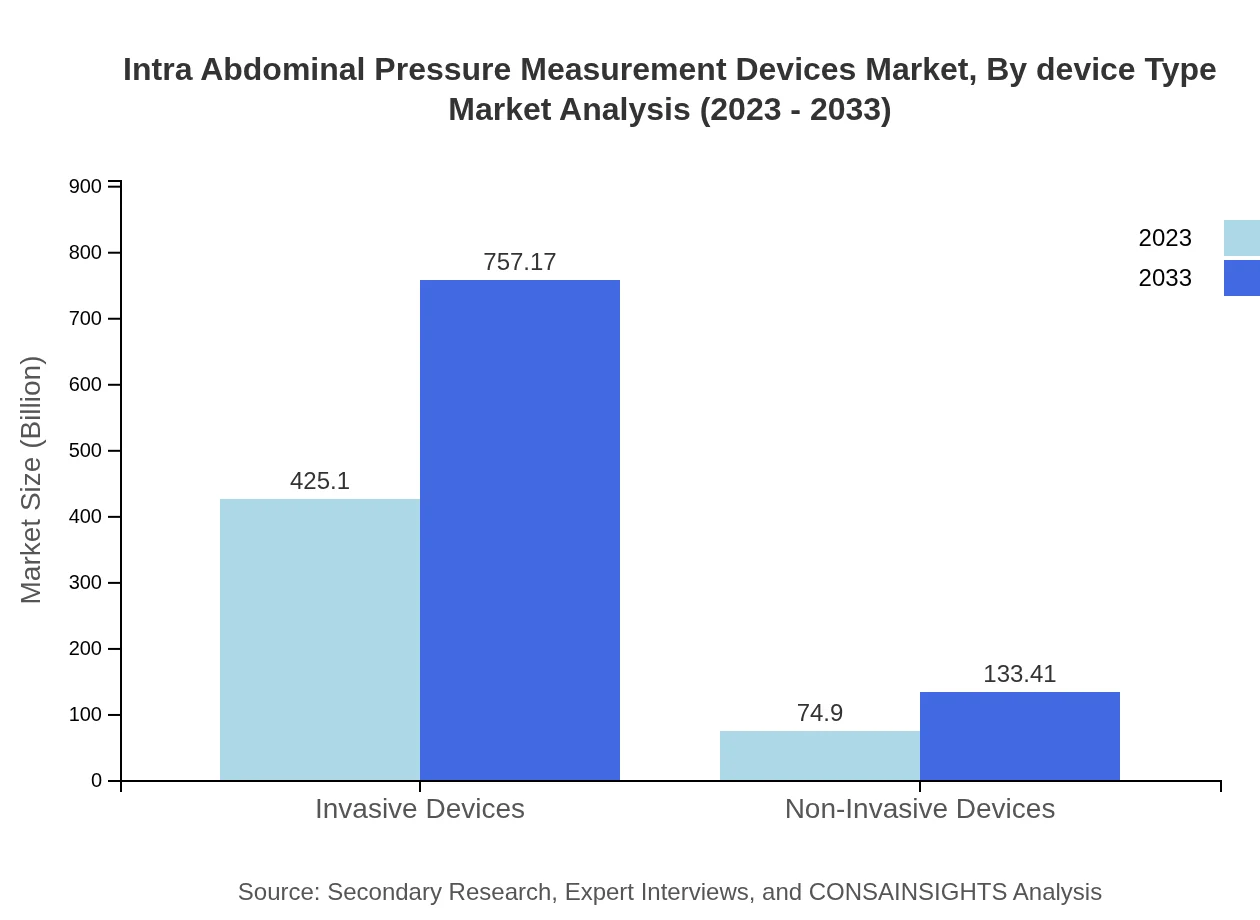
Invasive devices lead the market, with a size of $425.10 million in 2023 and projected growth to $757.17 million by 2033, capturing 85.02% market share. Non-invasive devices stand at $74.90 million in 2023, expected to increase to $133.41 million by 2033, representing a 14.98% market share, preferred for less invasive monitoring techniques.
Intra Abdominal Pressure Measurement Devices Market Analysis By Application
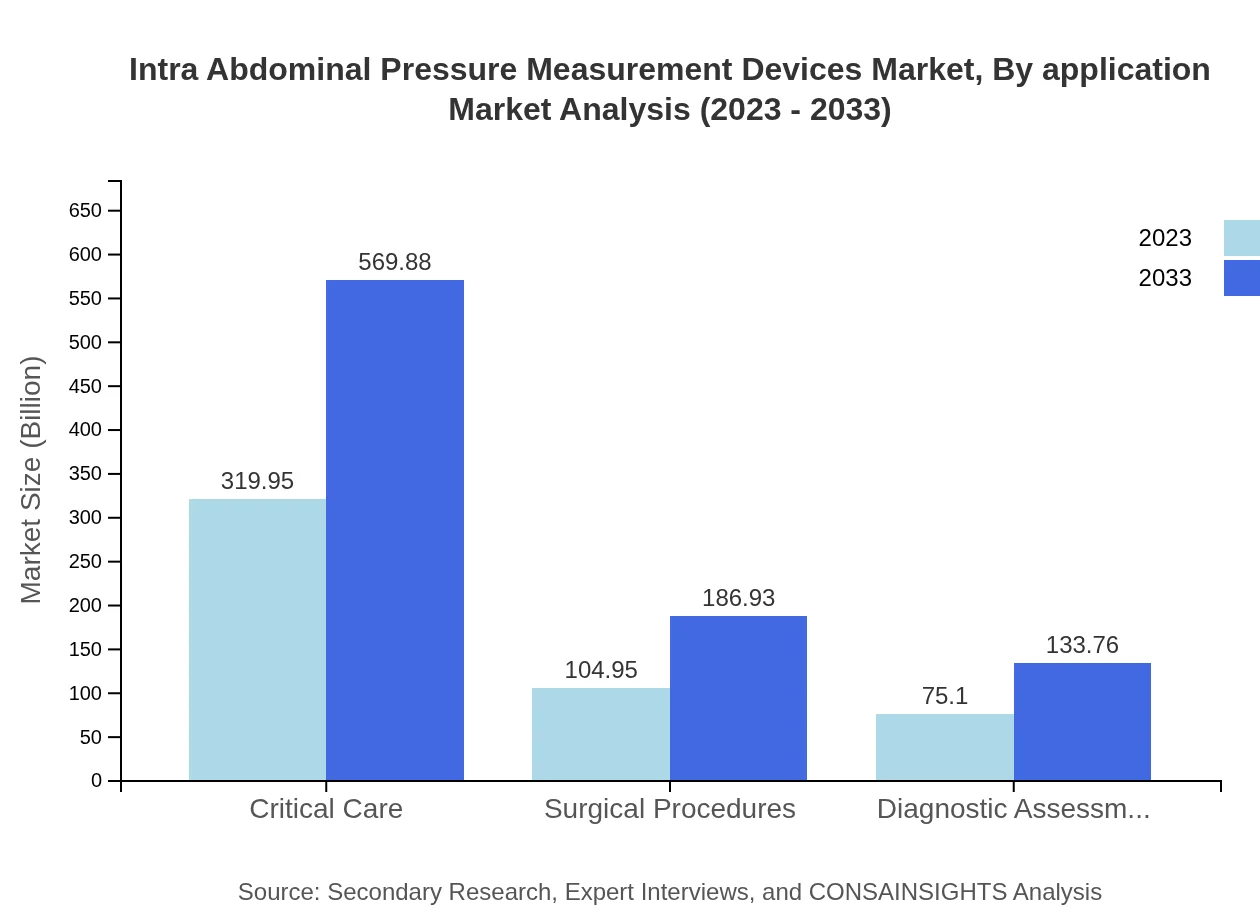
The critical care segment commands the market with a size of $319.95 million in 2023, forecasted to rise to $569.88 million by 2033. This segment is followed by surgical procedures at $104.95 million in 2023, expected to grow to $186.93 million by 2033, reflecting the growing need for intra-abdominal pressure measurement in hospital settings.
Intra Abdominal Pressure Measurement Devices Market Analysis By End User
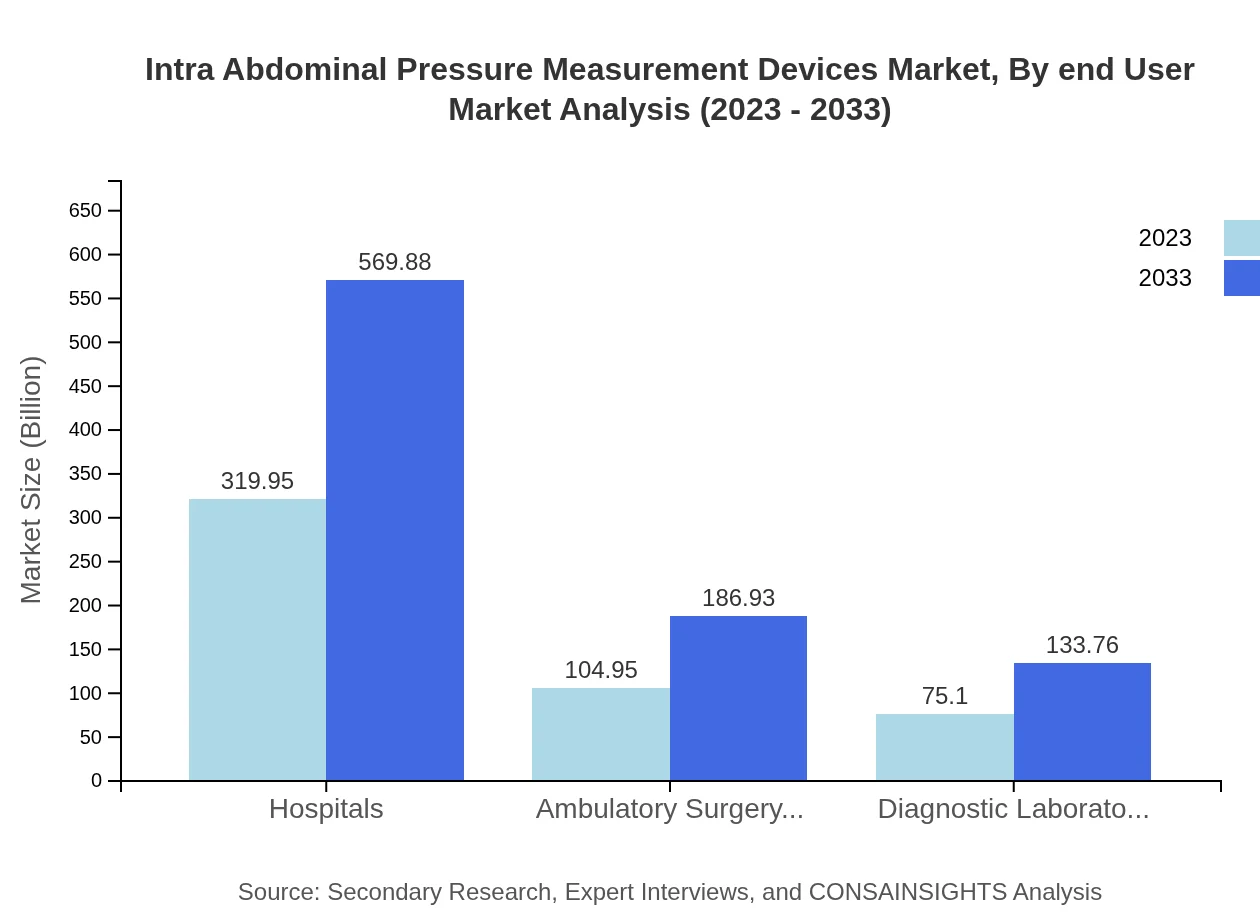
Hospitals are the significant end-users, accounting for $319.95 million in 2023 and projected to reach $569.88 million by 2033. Ambulatory surgery centers follow closely with a market size of $104.95 million in 2023, expected to grow to $186.93 million by 2033, reflecting their rising adoption of pressure measurement technology.
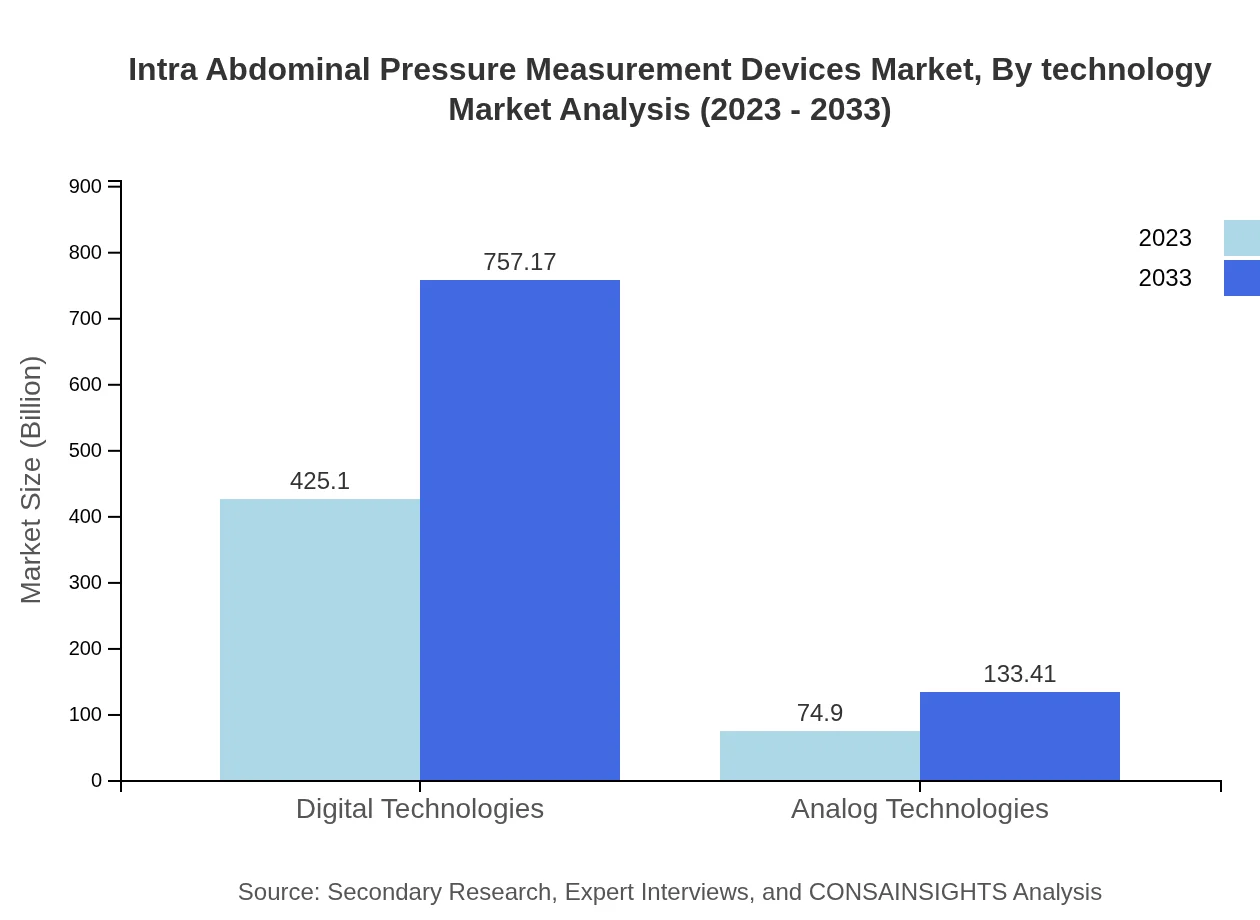
Intra Abdominal Pressure Measurement Devices Market Analysis By Technology
Digital technologies dominate the market, with a size of $425.10 million in 2023 and projected to grow to $757.17 million by 2033, capturing a significant share of 85.02%. In contrast, analog technologies represent a smaller segment, valued at $74.90 million in 2023 and expected to reach $133.41 million by 2033, indicating a gradual shift toward more advanced digital solutions.
Intra Abdominal Pressure Measurement Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Intra Abdominal Pressure Measurement Devices Industry
Medtronic :
A leading global healthcare solutions company, Medtronic develops innovative devices to monitor and manage intra-abdominal pressure, enhancing patient care quality in critical settings.Smiths Medical:
Specializing in medical devices, Smiths Medical offers precision solutions for intra-abdominal pressure monitoring, focusing on improving surgical outcomes and patient safety.C.R. Bard, a BD company:
C.R. Bard manufactures a wide range of medical products, including state-of-the-art intra-abdominal pressure measurement devices known for their reliability in critical care environments.Abbott Laboratories:
Abbott laboratories are known for their contributions to diagnostic device innovations, including those for pressure monitoring, contributing significantly to the advancement of patient management techniques.We're grateful to work with incredible clients.









FAQs
What is the market size of intra Abdominal Pressure Measurement Devices?
The global market size of intra-abdominal pressure measurement devices is currently estimated at approximately $500 million, with a forecasted Compound Annual Growth Rate (CAGR) of 5.8% over the coming years, indicating steady growth in this critical healthcare segment.
What are the key market players or companies in this intra Abdominal Pressure Measurement Devices industry?
Key players in the intra-abdominal pressure measurement devices market include established medical device companies focusing on innovative monitoring technologies, alongside emerging firms dedicated to advanced pressure measurement solutions, enhancing patient care in critical settings.
What are the primary factors driving the growth in the intra Abdominal Pressure Measurement Devices industry?
Growth in the intra-abdominal pressure measurement devices market is driven by rising incidences of abdominal disorders, increasing surgical procedures, advancements in medical technology, and the growing awareness of the importance of intra-abdominal pressure monitoring within healthcare.
Which region is the fastest Growing in the intra Abdominal Pressure Measurement Devices?
The fastest-growing region for intra-abdominal pressure measurement devices is expected to be North America, with the market size anticipated to grow from $174.25 million in 2023 to $310.37 million by 2033, fueled by increased healthcare funding and technological advancements.
Does ConsaInsights provide customized market report data for the intra Abdominal Pressure Measurement Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the intra-abdominal pressure measurement devices industry, allowing clients to access unique insights that align with their strategic objectives and market inquiries.
What deliverables can I expect from this intra Abdominal Pressure Measurement Devices market research project?
Deliverables from our intra-abdominal pressure measurement devices market research project include comprehensive market analysis reports, insightful data visualizations, competitive landscape assessments, and strategic recommendations tailored to enhance decision-making in the industry.
What are the market trends of intra Abdominal Pressure Measurement Devices?
Market trends in intra-abdominal pressure measurement devices include a shift towards digital technologies for improved accuracy and real-time monitoring, increased investment in healthcare infrastructure, and a growing focus on non-invasive methods to enhance patient comfort and safety.