Intraosseous Infusion Device Market Report
Published Date: 31 January 2026 | Report Code: intraosseous-infusion-device
Intraosseous Infusion Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Intraosseous Infusion Device market from 2023 to 2033. Insights include market size, trends, segmentation, regional analysis, and leading companies, aiming to furnish stakeholders with comprehensive data for strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
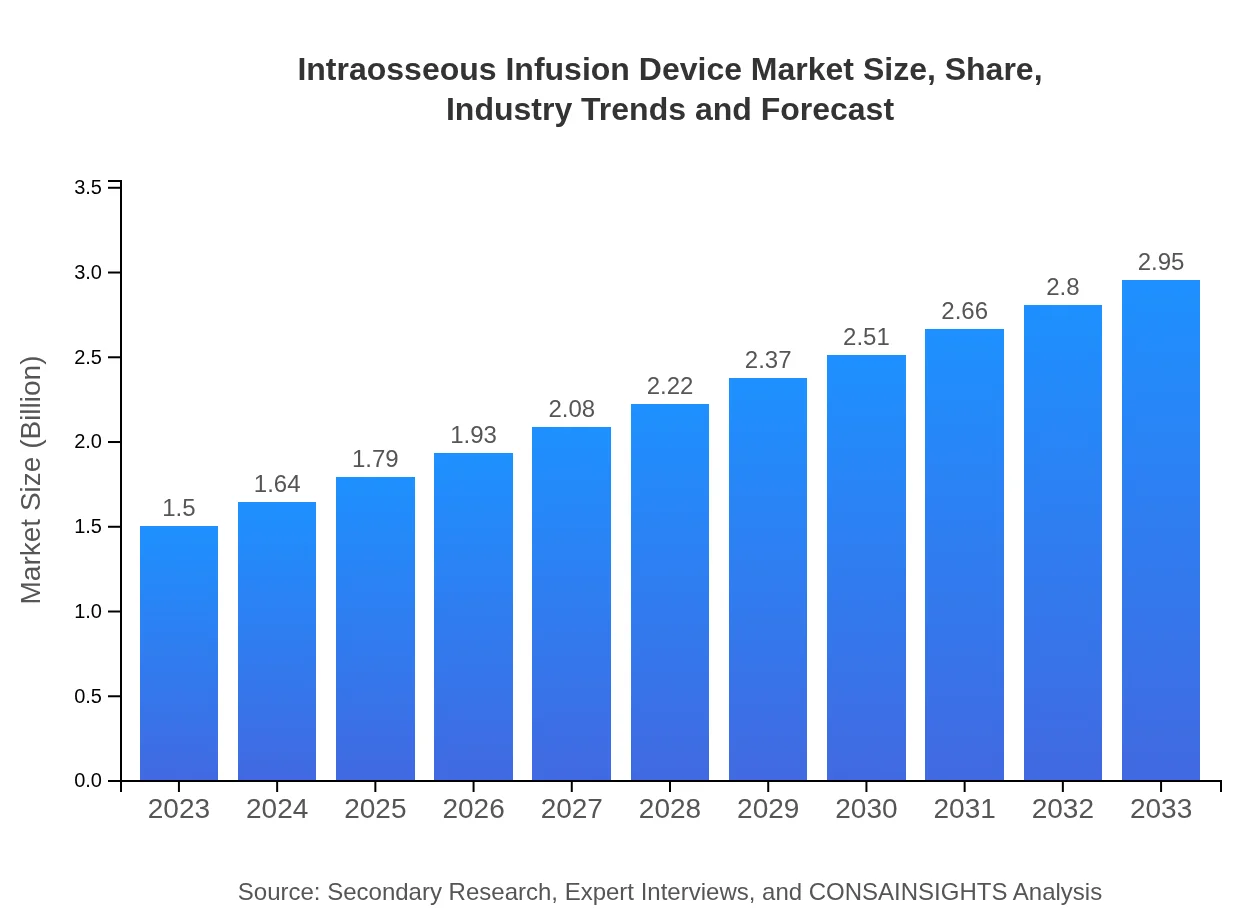
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | BD (Becton, Dickinson and Company), Zoll Medical Corporation, Pyng Medical Corp. |
| Last Modified Date | 31 January 2026 |
Intraosseous Infusion Device Market Overview
Customize Intraosseous Infusion Device Market Report market research report
- ✔ Get in-depth analysis of Intraosseous Infusion Device market size, growth, and forecasts.
- ✔ Understand Intraosseous Infusion Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Intraosseous Infusion Device
What is the Market Size & CAGR of the Intraosseous Infusion Device market in 2023?
Intraosseous Infusion Device Industry Analysis
Intraosseous Infusion Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Intraosseous Infusion Device Market Analysis Report by Region
Europe Intraosseous Infusion Device Market Report:
Europe's market is expected to grow from USD 0.50 billion in 2023 to USD 0.99 billion by 2033, fueled by the increasing adoption of advanced medical technology and government initiatives supporting emergency medical care.Asia Pacific Intraosseous Infusion Device Market Report:
In 2023, the Asia Pacific region accounts for a market size of USD 0.25 billion, anticipated to reach USD 0.50 billion by 2033. The growth is primarily driven by increasing investments in healthcare infrastructure and rising trauma cases in densely populated areas.North America Intraosseous Infusion Device Market Report:
In North America, the market is valued at USD 0.54 billion in 2023 and is expected to expand to USD 1.06 billion by 2033. The region's growth is bolstered by advanced healthcare facilities, robust emergency medical services, and high awareness levels concerning intraosseous infusion procedures.South America Intraosseous Infusion Device Market Report:
The South American Intraosseous Infusion Device market is projected to grow from USD 0.07 billion in 2023 to USD 0.13 billion by 2033. Challenges regarding healthcare accessibility and resource allocation hinder rapid growth; however, increasing healthcare initiatives are improving the scenario.Middle East & Africa Intraosseous Infusion Device Market Report:
This region holds a market size of USD 0.14 billion in 2023, with projections of reaching USD 0.27 billion by 2033. The growth is propelled by improving healthcare infrastructure and rising initiatives to address emergency medical care challenges.Tell us your focus area and get a customized research report.
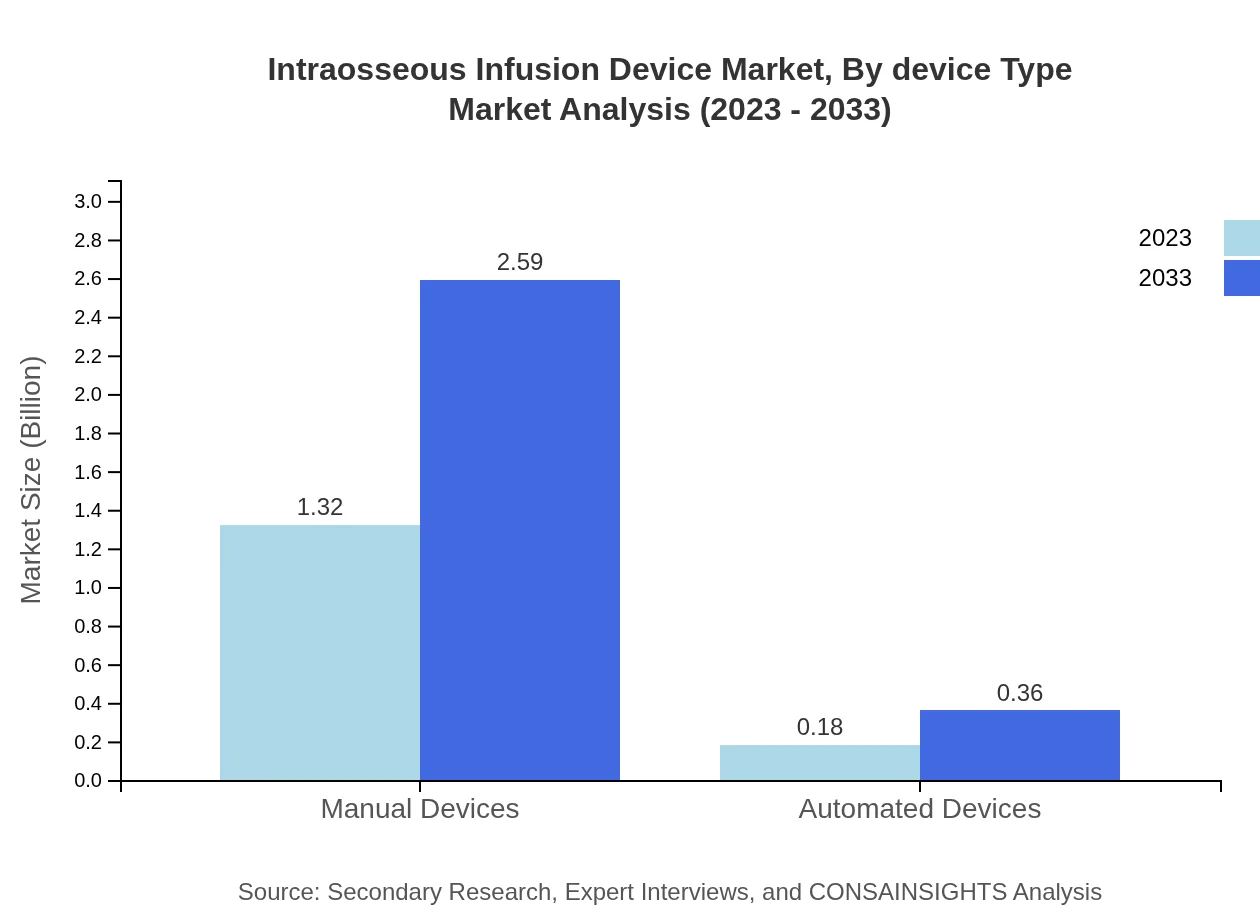
Intraosseous Infusion Device Market Analysis By Device Type
The market is segmented into manual and automated intraosseous devices. Manual devices accounted for a significant size of USD 1.32 billion in 2023 and are projected to reach USD 2.59 billion by 2033. Automated devices, while smaller in scale, are witnessing increased adoption to enhance procedural efficiency and safety.
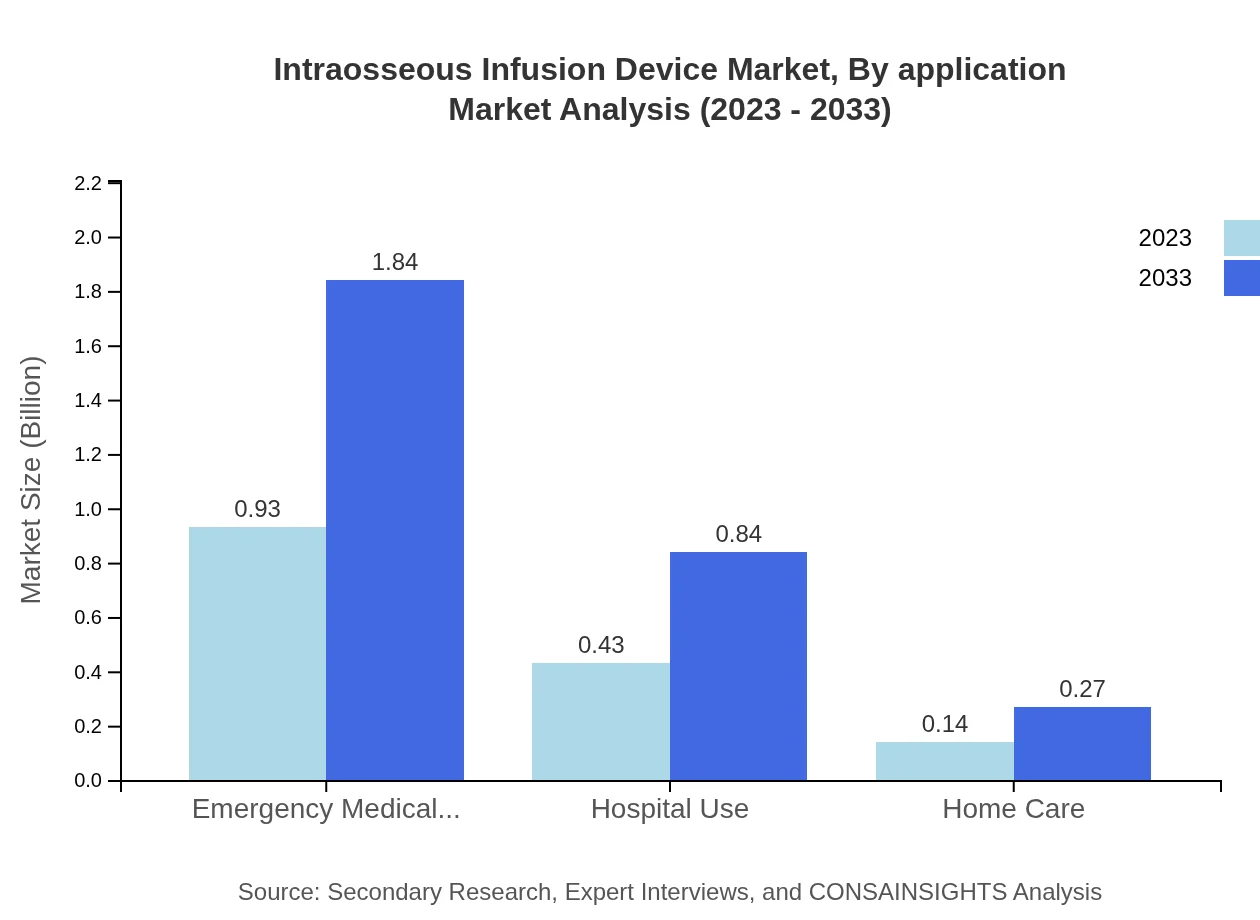
Intraosseous Infusion Device Market Analysis By Application
Applications for intraosseous infusion devices include trauma management, postoperative care, and emergency use. The trauma management segment is set to dominate the market, supported by increasing trauma cases and the need for rapid access to vein systems.
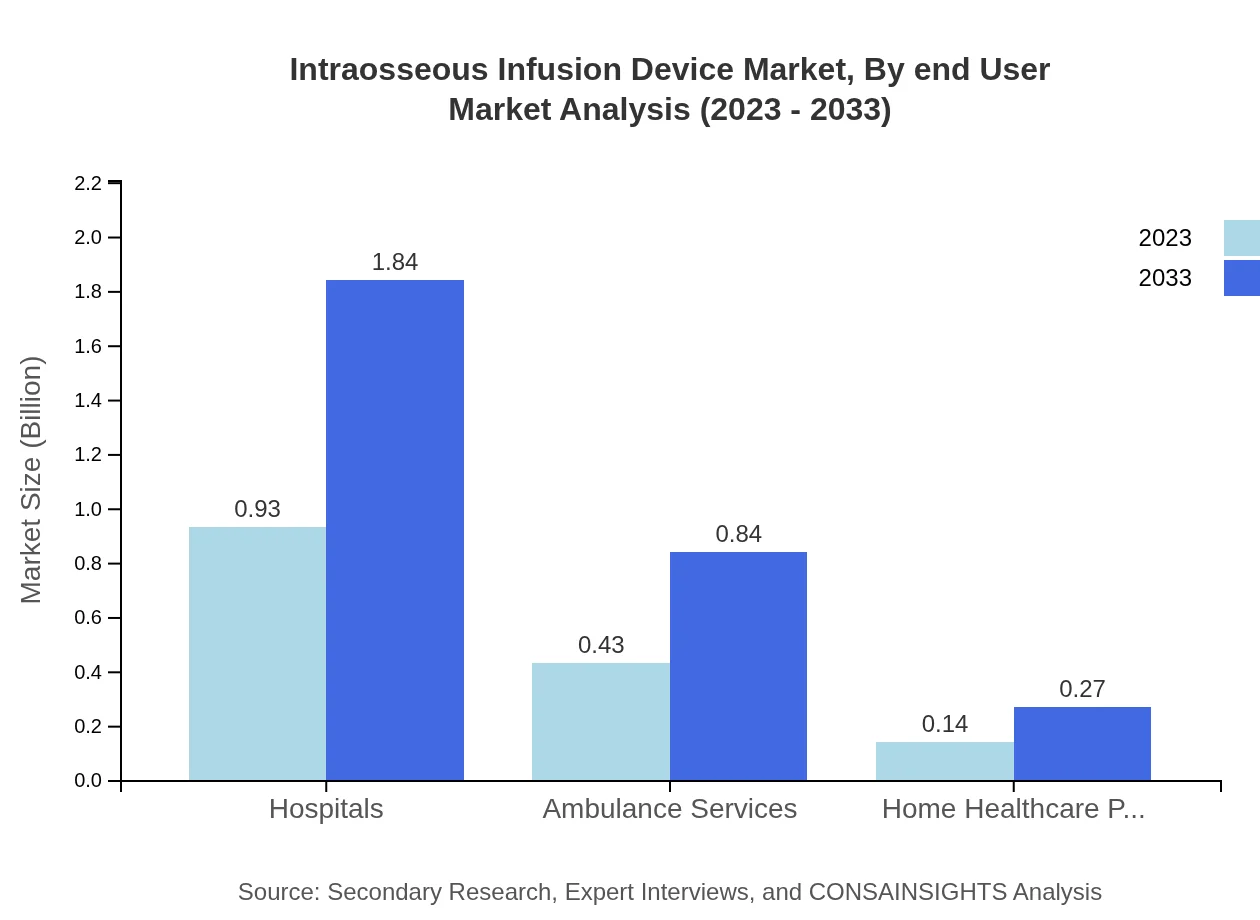
Intraosseous Infusion Device Market Analysis By End User
The primary end-users include hospitals, ambulance services, and home healthcare providers. Hospitals represent the largest segment, expected to grow from USD 0.93 billion to USD 1.84 billion by 2033, due to high demand for effective medical care and rising patient volumes.
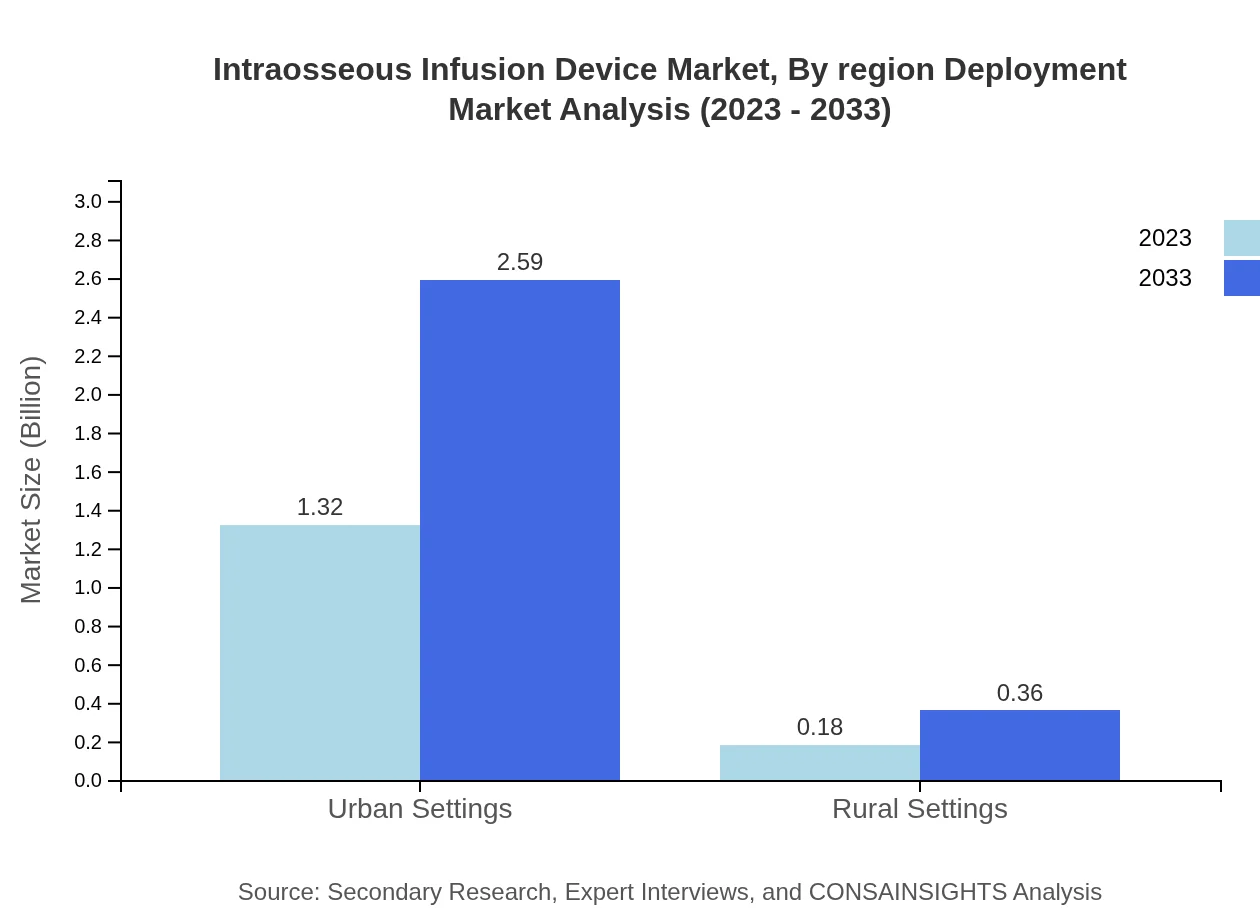
Intraosseous Infusion Device Market Analysis By Region Deployment
Regional deployment highlights the popularity of intraosseous devices in urban settings, which accounted for a market size of USD 1.32 billion in 2023. In contrast, rural settings represented a smaller share, with anticipated growth driven by increasing access to emergency care.
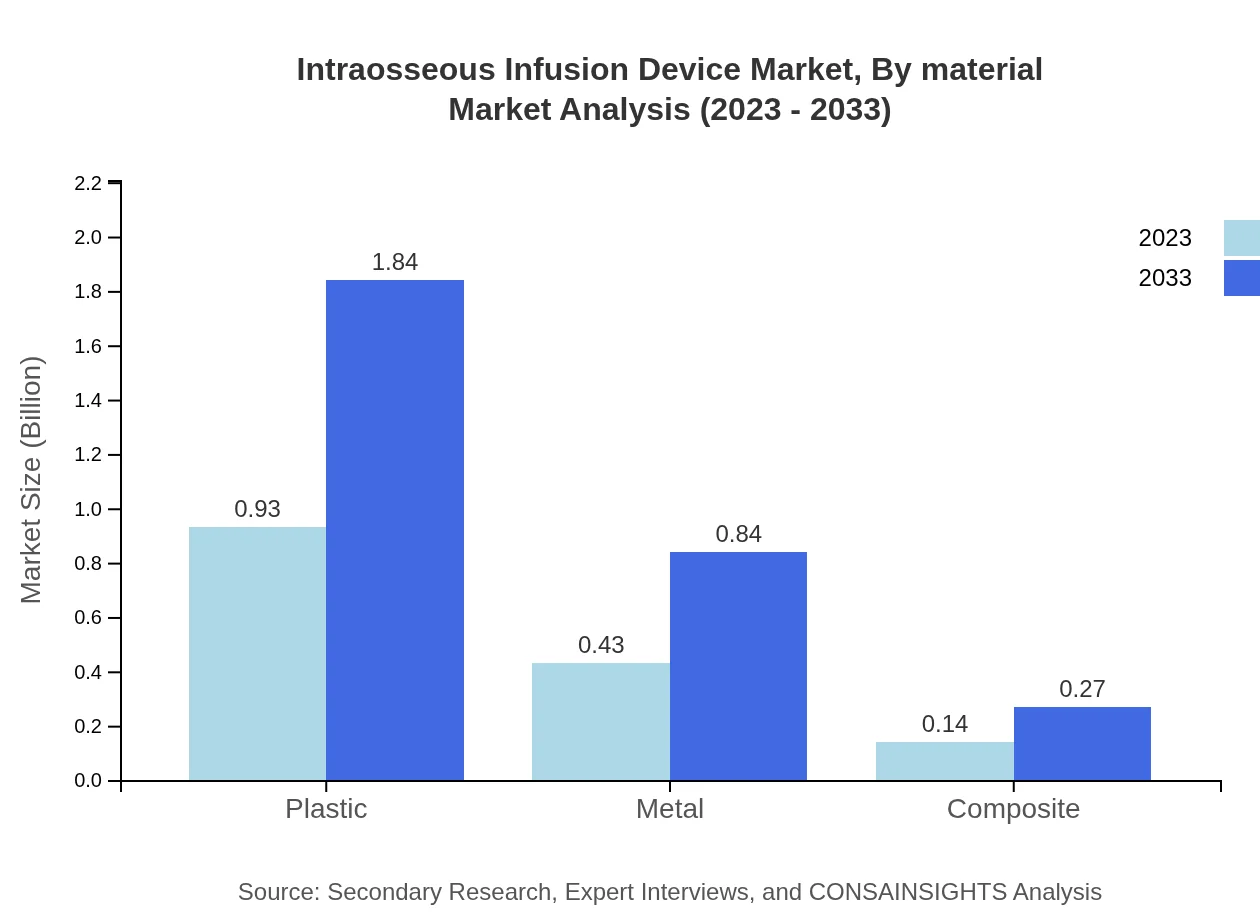
Intraosseous Infusion Device Market Analysis By Material
The market materials include plastic, metal, and composite devices. Plastic devices dominate, expected to grow alongside advancements in material technology providing lightweight and design-enhanced options for better user experience.
Intraosseous Infusion Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Intraosseous Infusion Device Industry
BD (Becton, Dickinson and Company):
A leading global medical technology company, BD focuses on advancing drug delivery and improving outcomes through innovative intraosseous infusion devices.Zoll Medical Corporation:
Zoll specializes in medical devices and software, providing emergency medical services with advanced intraosseous infusion solutions for more effective patient treatment.Pyng Medical Corp.:
Known for its specialized medical devices, Pyng's intraosseous infusion technology has gained recognition in emergency medicine across different healthcare settings.We're grateful to work with incredible clients.









FAQs
What is the market size of intraosseous Infusion Device?
The intraosseous infusion device market is expected to reach $1.5 billion by 2033, growing at a CAGR of 6.8%. This growth reflects a significant demand for efficient trauma care and emergency responses globally.
What are the key market players or companies in this intraosseous Infusion Device industry?
Key players in the intraosseous infusion device industry include major medical device manufacturers that specialize in emergency medical equipment. Their innovations drive market growth, allowing for more efficient medical responses in trauma situations.
What are the primary factors driving the growth in the intraosseous Infusion Device industry?
Growth in the intraosseous infusion device market is driven by increasing emergency medical services, advancements in medical technology, and rising awareness about effective trauma treatment measures among healthcare professionals.
Which region is the fastest Growing in the intraosseous Infusion Device?
North America is the fastest-growing region in the intraosseous infusion device market, expected to reach $1.06 billion by 2033. This growth is fueled by a robust healthcare infrastructure and increasing adoption in emergency medical services.
Does ConsaInsights provide customized market report data for the intraosseous Infusion Device industry?
Yes, ConsaInsights offers customized market report data for the intraosseous infusion device industry. Clients can obtain tailored insights that meet specific business needs and strategic objectives.
What deliverables can I expect from this intraosseous Infusion Device market research project?
From the intraosseous infusion device market research project, expect detailed market analysis reports, comprehensive data on regional trends, competitor analysis, and forecasts segmented by type and application.
What are the market trends of intraosseous Infusion Device?
Current trends in the intraosseous infusion device market include a shift towards automated devices, increasing use in diverse healthcare settings, and innovations aimed at enhancing patient safety and treatment efficiency.