Intrapartum Monitoring Devices Market Report
Published Date: 31 January 2026 | Report Code: intrapartum-monitoring-devices
Intrapartum Monitoring Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Intrapartum Monitoring Devices market from 2023 to 2033. It includes market size forecasts, regional analysis, industry trends, and key players, offering valuable data for stakeholders looking to understand or invest in this sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
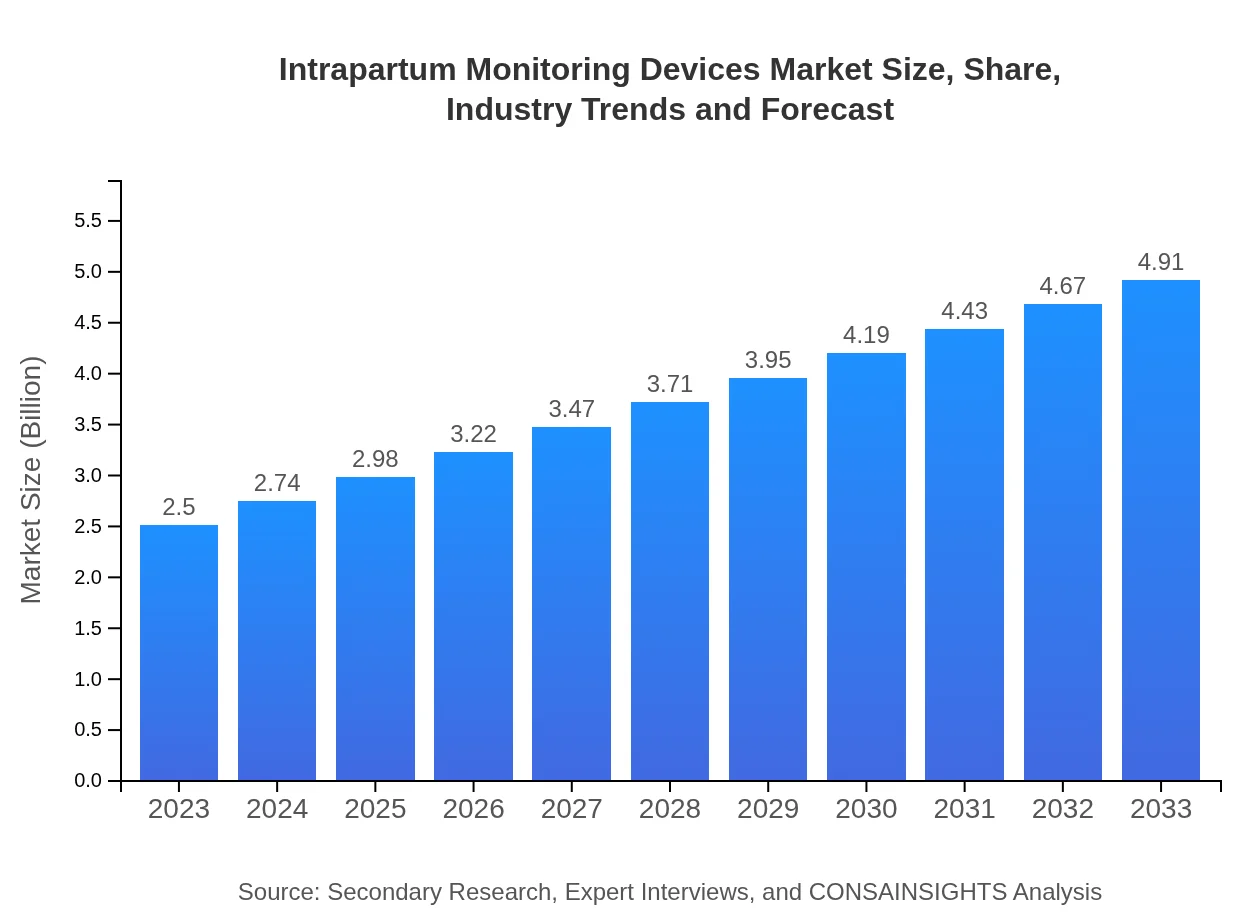
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Philips Healthcare, GE Healthcare, Natus Medical Incorporated, Fujifilm Medical Systems, Medtronic |
| Last Modified Date | 31 January 2026 |
Intrapartum Monitoring Devices Market Overview
Customize Intrapartum Monitoring Devices Market Report market research report
- ✔ Get in-depth analysis of Intrapartum Monitoring Devices market size, growth, and forecasts.
- ✔ Understand Intrapartum Monitoring Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Intrapartum Monitoring Devices
What is the Market Size & CAGR of Intrapartum Monitoring Devices market in 2023?
Intrapartum Monitoring Devices Industry Analysis
Intrapartum Monitoring Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Intrapartum Monitoring Devices Market Analysis Report by Region
Europe Intrapartum Monitoring Devices Market Report:
The European market is valued at $0.64 billion in 2023, with projections of $1.26 billion by 2033. A strong focus on maternal health and safety, as well as an increase in the number of childbirths in hospitals and birthing centers, is driving growth. The region benefits from rigorous health regulations that ensure high-quality medical devices.Asia Pacific Intrapartum Monitoring Devices Market Report:
In the Asia Pacific region, the intrapartum monitoring devices market is valued at $0.52 billion in 2023 and is projected to grow to $1.02 billion by 2033. This growth is driven by rising healthcare spending, increasing births in healthcare facilities, and heightened awareness regarding maternal health. Countries like India and China are seeing rapid advancements in healthcare infrastructure, fueling demand for such devices.North America Intrapartum Monitoring Devices Market Report:
North America dominates the market, with a valuation of $0.92 billion in 2023 and a projected size of $1.80 billion by 2033. The presence of well-established healthcare infrastructure, high disposable incomes, and increasing technological advancements contribute to the market's expansion. The demand for advanced and connected health monitoring devices is also increasing among consumers.South America Intrapartum Monitoring Devices Market Report:
The South American market for intrapartum monitoring devices was valued at $0.13 billion in 2023 and is expected to reach $0.26 billion by 2033. Growth in this sector is supported by improving healthcare systems and government initiatives promoting maternal health. However, disparities in healthcare access in different countries may impact overall market performance.Middle East & Africa Intrapartum Monitoring Devices Market Report:
In the Middle East and Africa, the market is valued at $0.29 billion in 2023 and is anticipated to reach $0.58 billion by 2033. The growing number of maternal healthcare facilities and an increasing focus on improving healthcare quality are key drivers. However, the market faces challenges related to varying economic conditions and healthcare accessibility across the region.Tell us your focus area and get a customized research report.
Intrapartum Monitoring Devices Market Analysis By Device Type
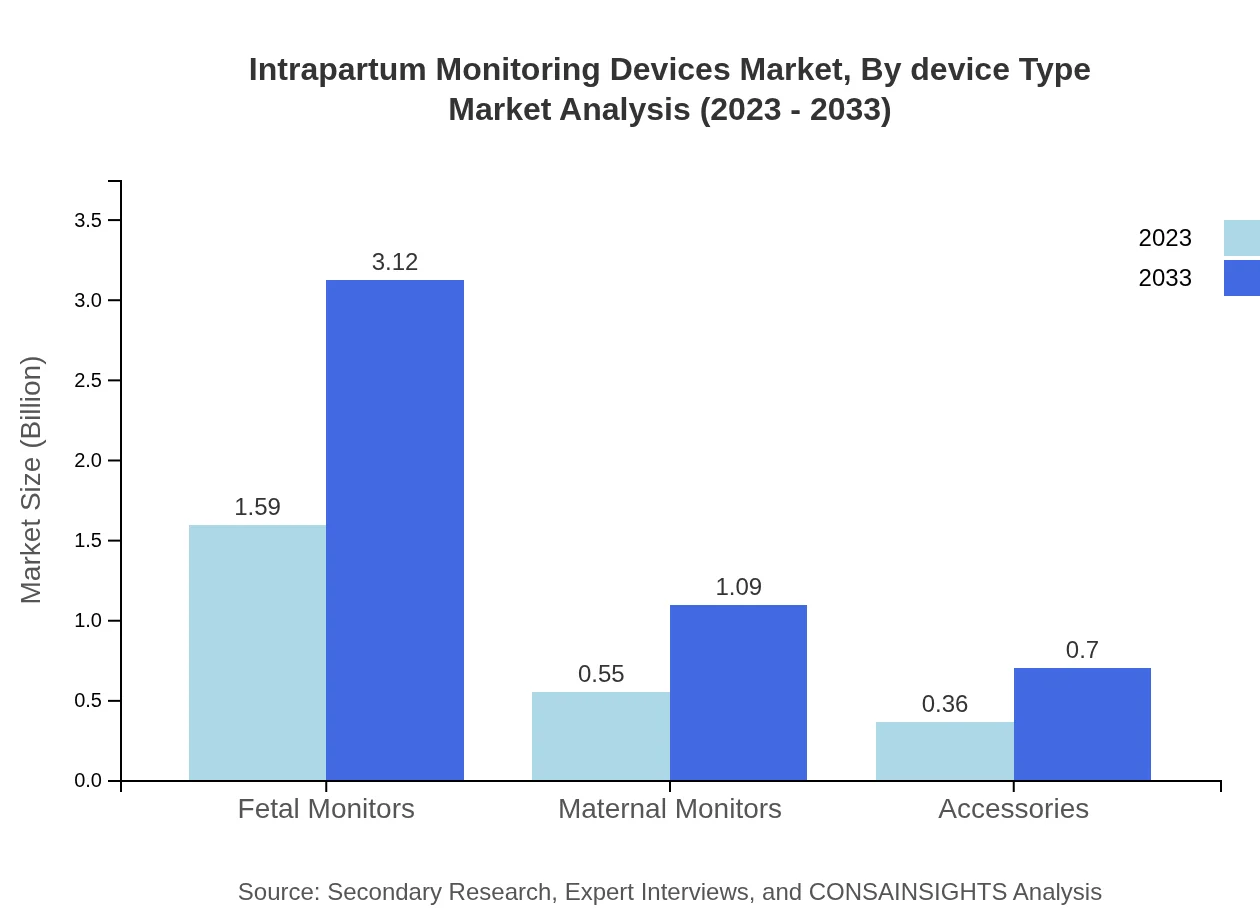
The segment focuses on various devices including fetal monitors, maternal monitors, and accessories. In 2023, fetal monitors dominate the market with a valuation of $1.59 billion, expected to grow to $3.12 billion by 2033. Maternal monitors and accessories follow, valued at $0.55 billion and $0.36 billion in 2023, forecasted to reach $1.09 billion and $0.70 billion, respectively.
Intrapartum Monitoring Devices Market Analysis By Technology
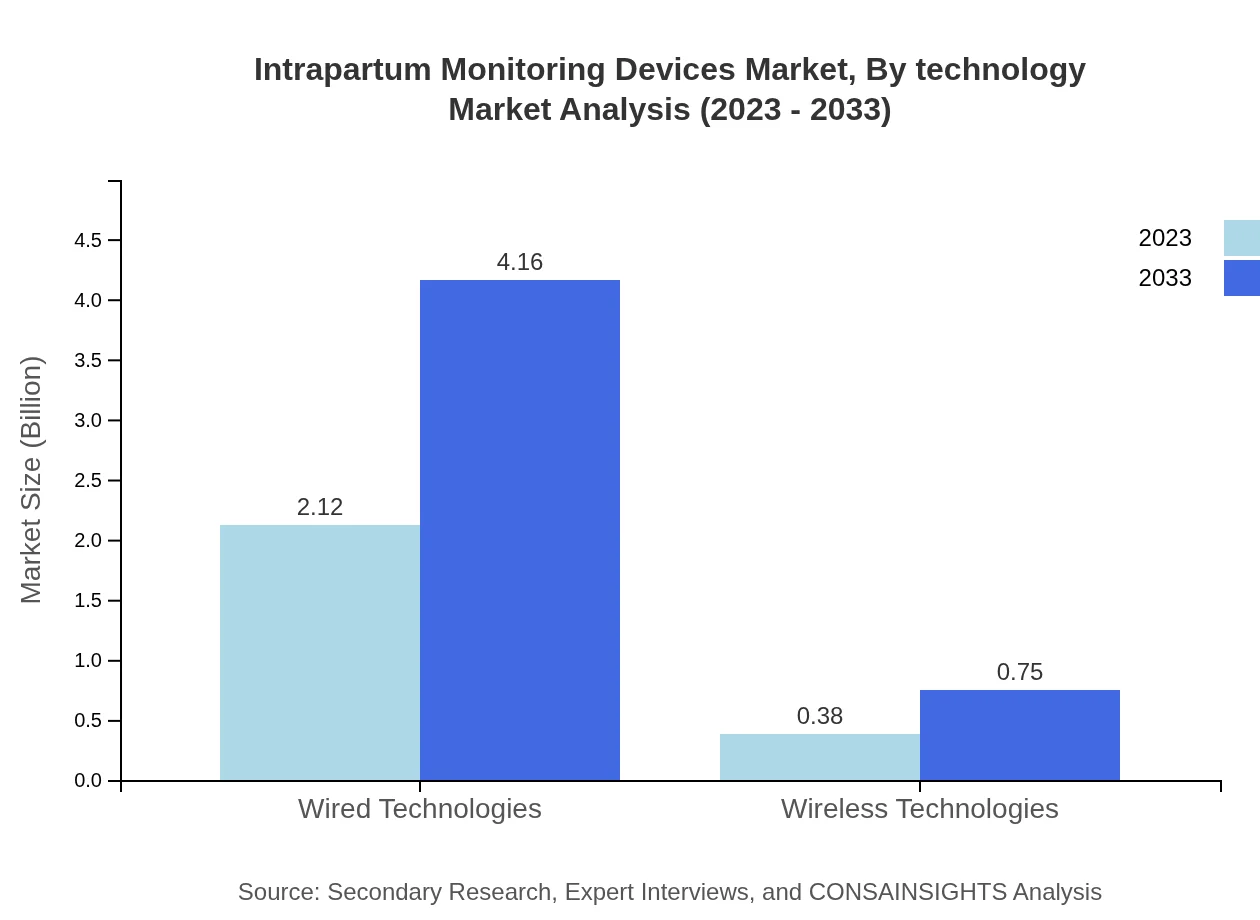
The market is segmented by technology into wired and wireless. Wired technologies lead, accounting for $2.12 billion in 2023, projected to ascend to $4.16 billion by 2033, while wireless technologies, valued at $0.38 billion, are expected to grow to $0.75 billion during the same period.
Intrapartum Monitoring Devices Market Analysis By Application
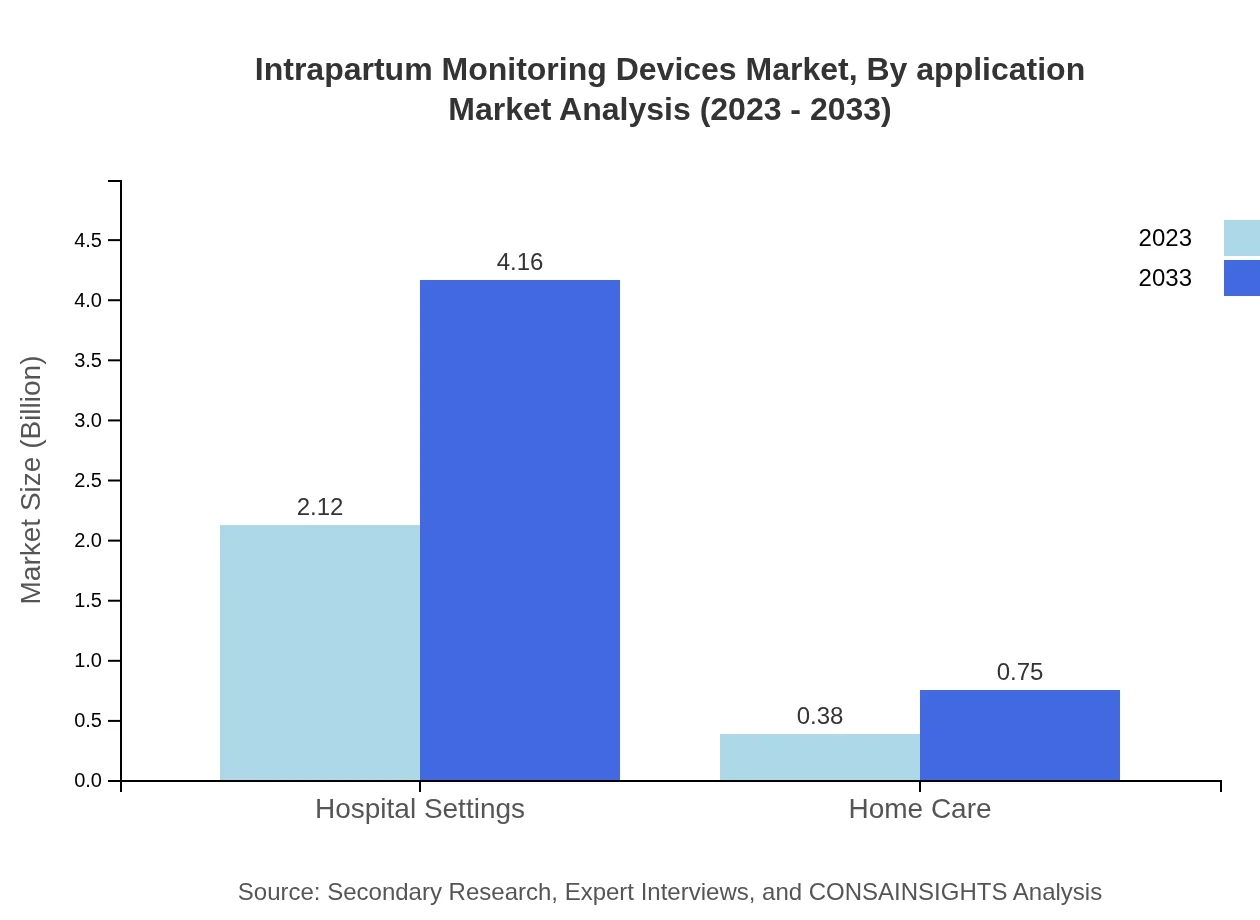
This segment includes hospital settings, birthing centers, and home care. Hospital settings dominate, with market size of $2.12 billion expected to rise to $4.16 billion by 2033. Birthing centers and home care markets are valued at $0.55 billion and $0.36 billion in 2023, expected to reach $1.09 billion and $0.70 billion, respectively.
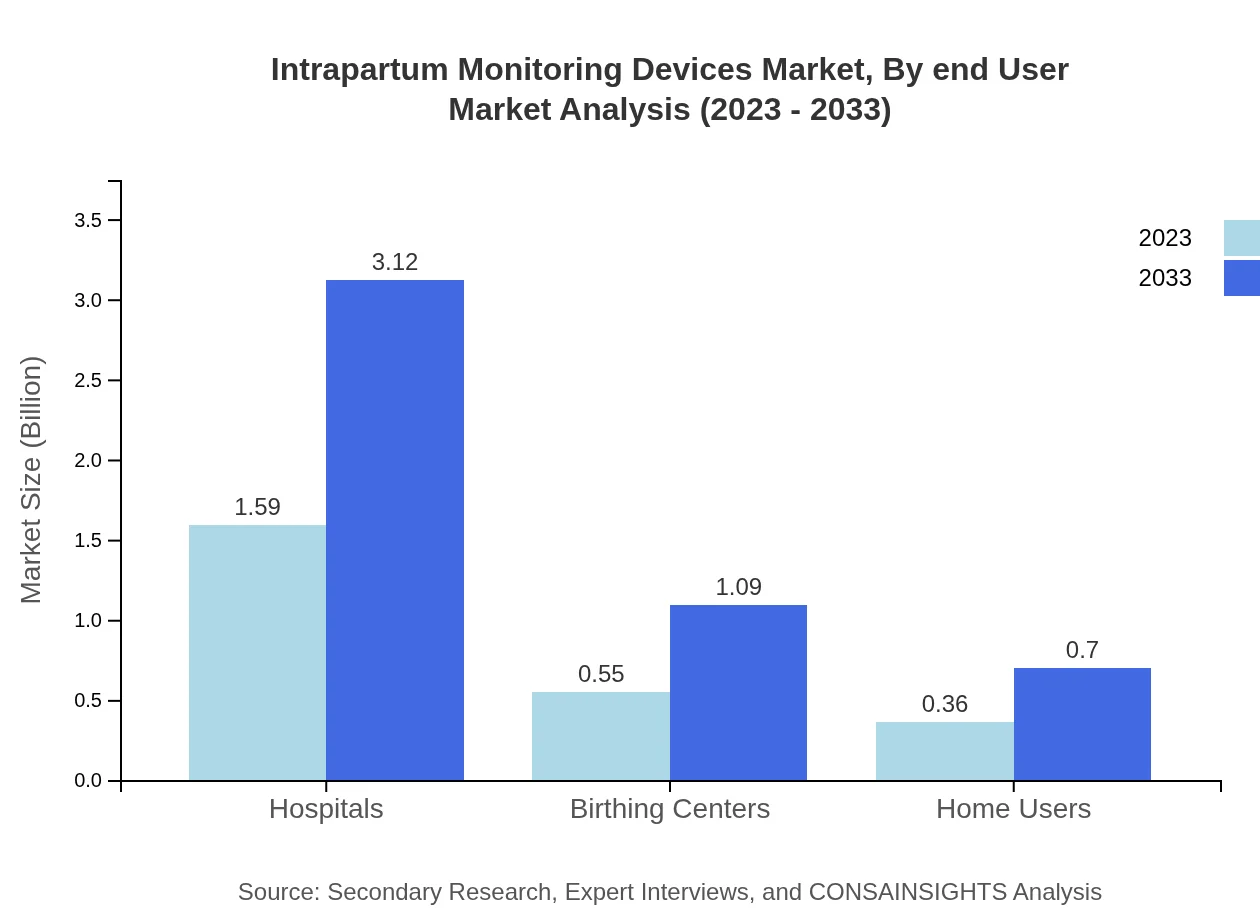
Intrapartum Monitoring Devices Market Analysis By End User
End-user segmentation indicates hospitals as the primary users with a market size of $1.59 billion in 2023, and anticipated growth to $3.12 billion by 2033. Birthing centers and home users are also significant end-users, with current market sizes of $0.55 billion and $0.36 billion, expected to grow accordingly.
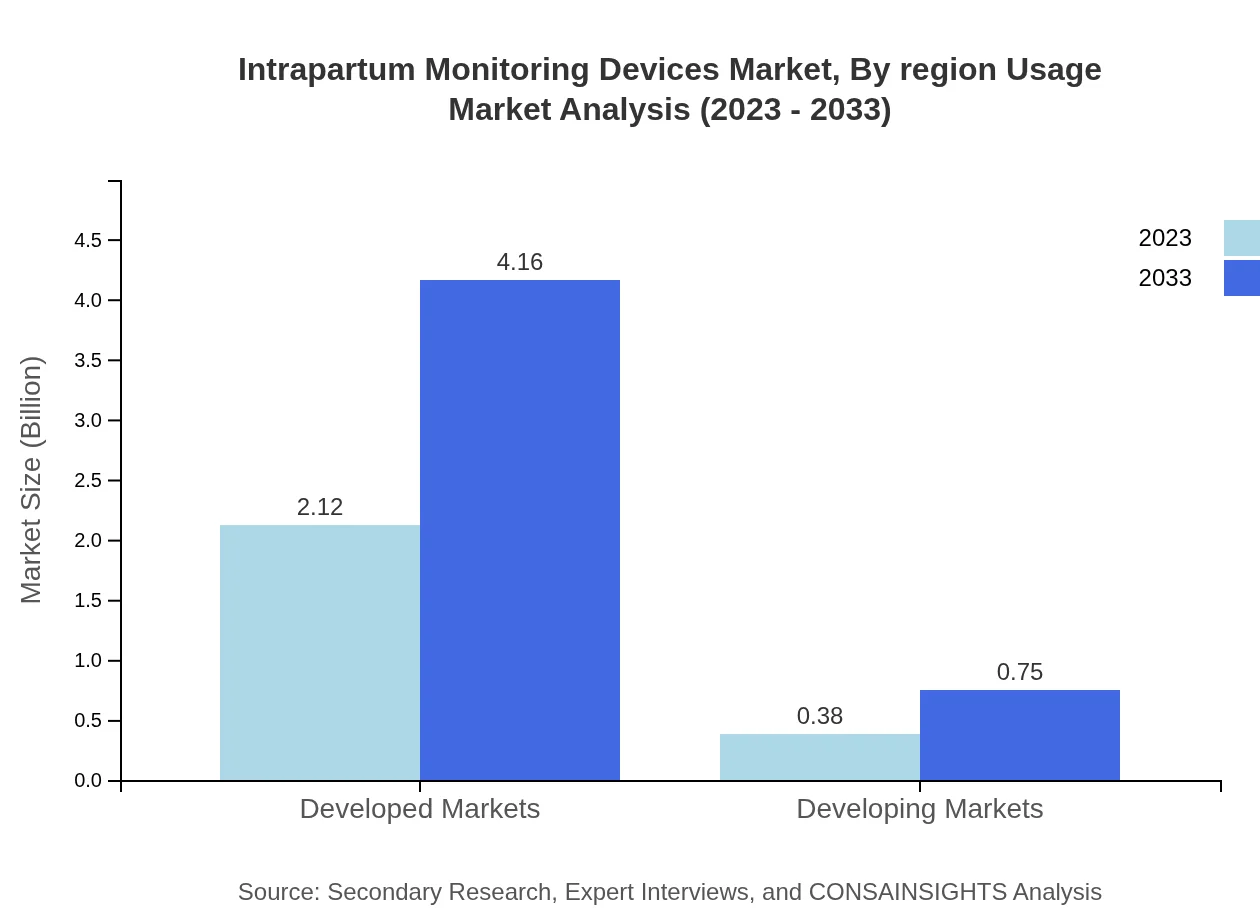
Intrapartum Monitoring Devices Market Analysis By Region Usage
Regions are crucial in market segmentation, with developed markets, particularly North America and Europe, leading in both growth and market share due to advanced healthcare systems. Emerging markets in Asia and Latin America show rapid growth potential due to healthcare improvements and increased healthcare expenditures.
Intrapartum Monitoring Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Intrapartum Monitoring Devices Industry
Philips Healthcare:
Philips is a leader in health technology, focusing on innovative solutions for patient monitoring, including intrapartum monitoring devices.GE Healthcare:
GE Healthcare offers a comprehensive suite of monitoring solutions that enhance maternal and neonatal care, contributing significantly to market growth.Natus Medical Incorporated:
Natus specializes in newborn and maternal care medical devices, and is recognized for its innovative intrapartum and neonatal monitoring solutions.Fujifilm Medical Systems:
Fujifilm provides advanced healthcare solutions focused on improving diagnostic imaging and real-time monitoring technologies.Medtronic :
Medtronic's extensive range of medical devices includes solutions for intrapartum monitoring, contributing to healthcare safety and quality.We're grateful to work with incredible clients.









FAQs
What is the market size of intrapartum Monitoring Devices?
The intrapartum monitoring devices market is estimated to be valued at $2.5 billion in 2023, with a projected growth rate of 6.8% CAGR, reaching substantial market size by 2033 as healthcare technology adoption increases and maternal monitoring needs rise.
What are the key market players or companies in this intrapartum Monitoring Devices industry?
Key players in the intrapartum monitoring devices industry include established medical technology companies specializing in maternal and fetal health. These companies invest heavily in R&D to innovate and expand their product lines, adapting to evolving healthcare needs.
What are the primary factors driving the growth in the intrapartum Monitoring Devices industry?
Key growth factors for the intrapartum monitoring devices market include rising awareness of maternal health, technological advancements in monitoring devices, increasing incidence of high-risk pregnancies, and the push for improved fetal health outcomes across healthcare facilities.
Which region is the fastest Growing in the intrapartum Monitoring Devices?
Asia Pacific emerges as the fastest-growing region in the intrapartum monitoring devices market, with market growth projected to rise from $0.52 billion in 2023 to $1.02 billion by 2033, driven by increasing healthcare investments and maternal healthcare initiatives.
Does ConsaInsights provide customized market report data for the intrapartum Monitoring Devices industry?
Yes, Consainsights offers customized market report data tailored to specific client needs in the intrapartum monitoring devices industry, allowing stakeholders to gain insights into niche markets and consumer behavior for more informed decision-making.
What deliverables can I expect from this intrapartum Monitoring Devices market research project?
Clients can expect detailed market analysis reports including market size estimations, segment breakdowns, competitive landscape reviews, growth forecasts, and insights on regional trends, all tailored to the unique aspects of the intrapartum monitoring devices sector.
What are the market trends of intrapartum Monitoring Devices?
Market trends in intrapartum monitoring devices include the rise of wireless technology, increased use of fetal and maternal monitors in hospitals and care settings, and a growing focus on smart and connected devices enhancing patient care and monitoring efficiency.