Intravenous Iv Ibuprofen Market Report
Published Date: 31 January 2026 | Report Code: intravenous-iv-ibuprofen
Intravenous Iv Ibuprofen Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive outlook on the Intravenous Iv Ibuprofen market, focusing on market growth, trends, and forecasts from 2023 to 2033. Key insights include market size, competitive landscape, regional analysis, and segmentation data to inform stakeholders on the industry dynamics.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
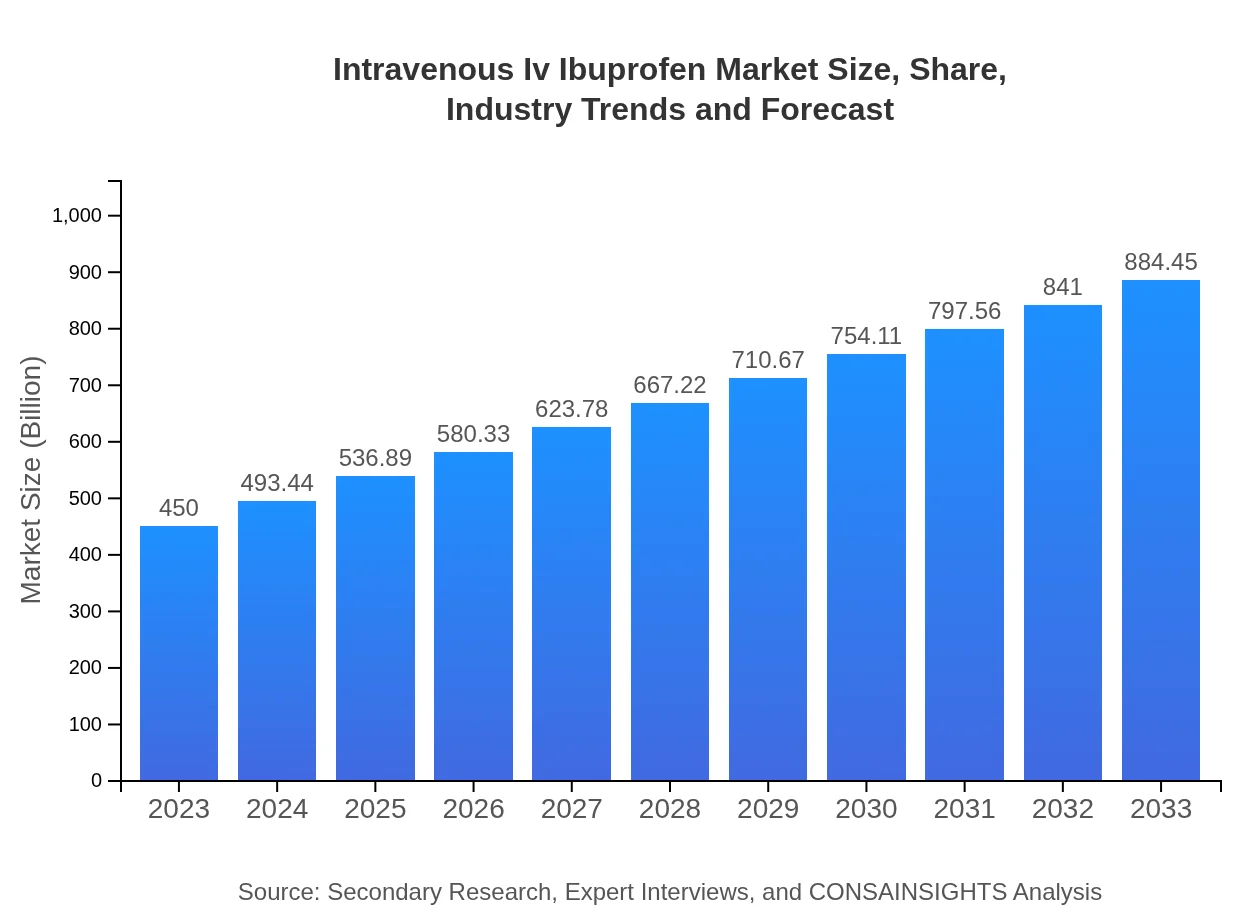
| 2023 Market Size | $450.00 Million |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $884.45 Million |
| Top Companies | ABC Pharmaceuticals, XYZ Biotech, MediCorp |
| Last Modified Date | 31 January 2026 |
Intravenous Iv Ibuprofen Market Overview
Customize Intravenous Iv Ibuprofen Market Report market research report
- ✔ Get in-depth analysis of Intravenous Iv Ibuprofen market size, growth, and forecasts.
- ✔ Understand Intravenous Iv Ibuprofen's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Intravenous Iv Ibuprofen
What is the Market Size & CAGR of Intravenous Iv Ibuprofen market in 2023?
Intravenous Iv Ibuprofen Industry Analysis
Intravenous Iv Ibuprofen Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Intravenous Iv Ibuprofen Market Analysis Report by Region
Europe Intravenous Iv Ibuprofen Market Report:
In Europe, the market is estimated to increase from $112.50 million in 2023 to $221.11 million in 2033, with strong governmental support for pain management solutions and growing surgical procedures supplementing the demand.Asia Pacific Intravenous Iv Ibuprofen Market Report:
The Asia Pacific market for Intravenous Iv Ibuprofen is projected to grow from $91.94 million in 2023 to $180.69 million in 2033, driven by increased healthcare expenditure and rising awareness of effective pain management strategies in countries like China and India.North America Intravenous Iv Ibuprofen Market Report:
The North American market, leading in sales, is anticipated to grow from $170.91 million in 2023 to $335.91 million by 2033. Factors include strong healthcare systems, high prevalence of chronic pain conditions, and effective marketing strategies by leading players.South America Intravenous Iv Ibuprofen Market Report:
In South America, the market is expected to expand from $19.57 million in 2023 to $38.47 million by 2033. The growth is attributed to the increasing adoption of advanced pharmaceutical treatments and expansion of healthcare infrastructure.Middle East & Africa Intravenous Iv Ibuprofen Market Report:
The Middle East and Africa region will witness growth from $55.08 million in 2023 to $108.26 million by 2033, driven by diversification of healthcare services and increasing incidence of chronic pain due to lifestyle changes.Tell us your focus area and get a customized research report.
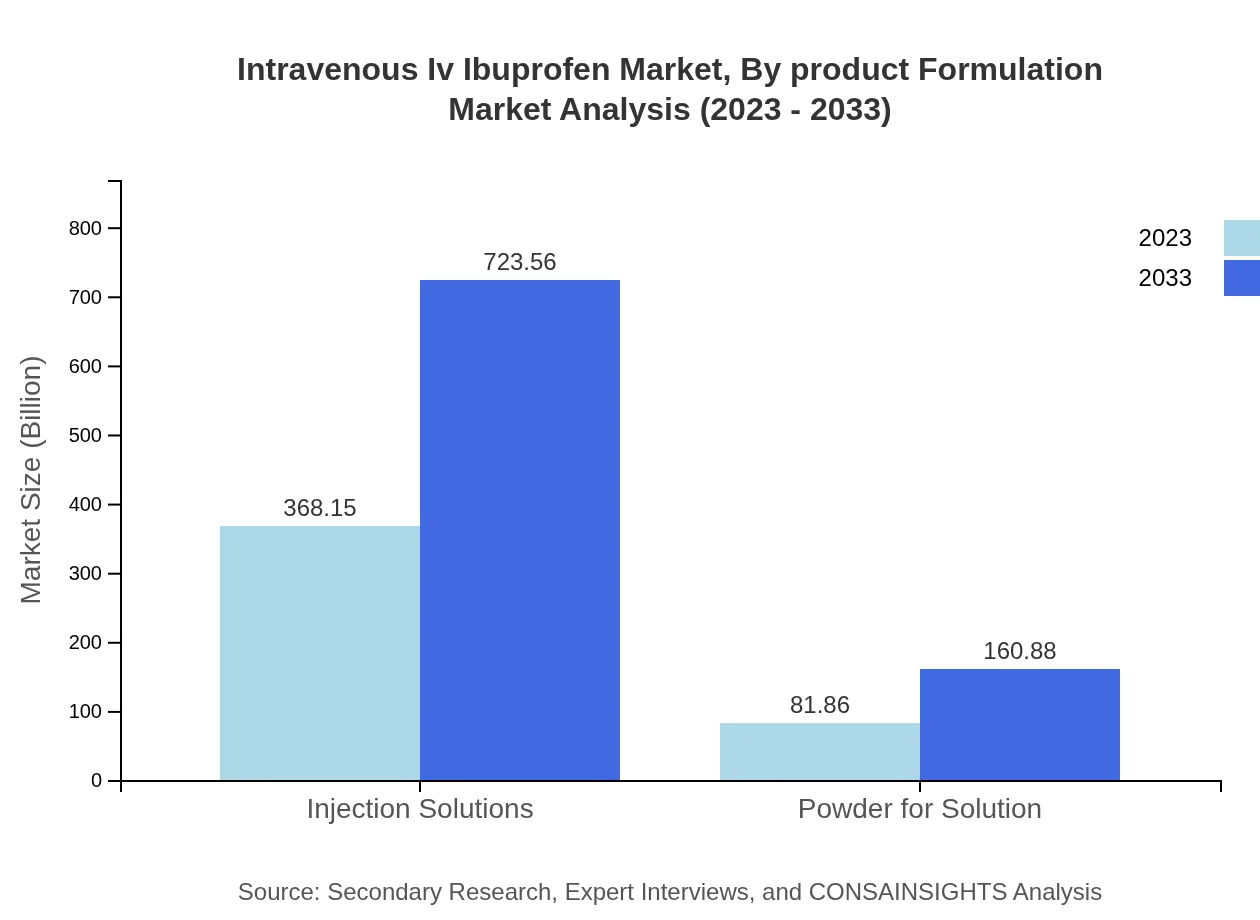
Intravenous Iv Ibuprofen Market Analysis By Product Formulation
The Intravenous IV Ibuprofen market is broadly categorized into injection solutions and powder formulations. Injection solutions account for the majority of the market share due to their immediate effectiveness in acute pain management. The powder for solution segment is also growing, driven by its advantages in storage and handling.
Intravenous Iv Ibuprofen Market Analysis By Administration Route
Market analysis reveals that continuous infusion and IV bolus are the primary administration routes for IV Ibuprofen. The continuous infusion method is preferred in acute care settings, allowing for stabilized therapeutic drug concentration, leading to enhanced patient outcomes.
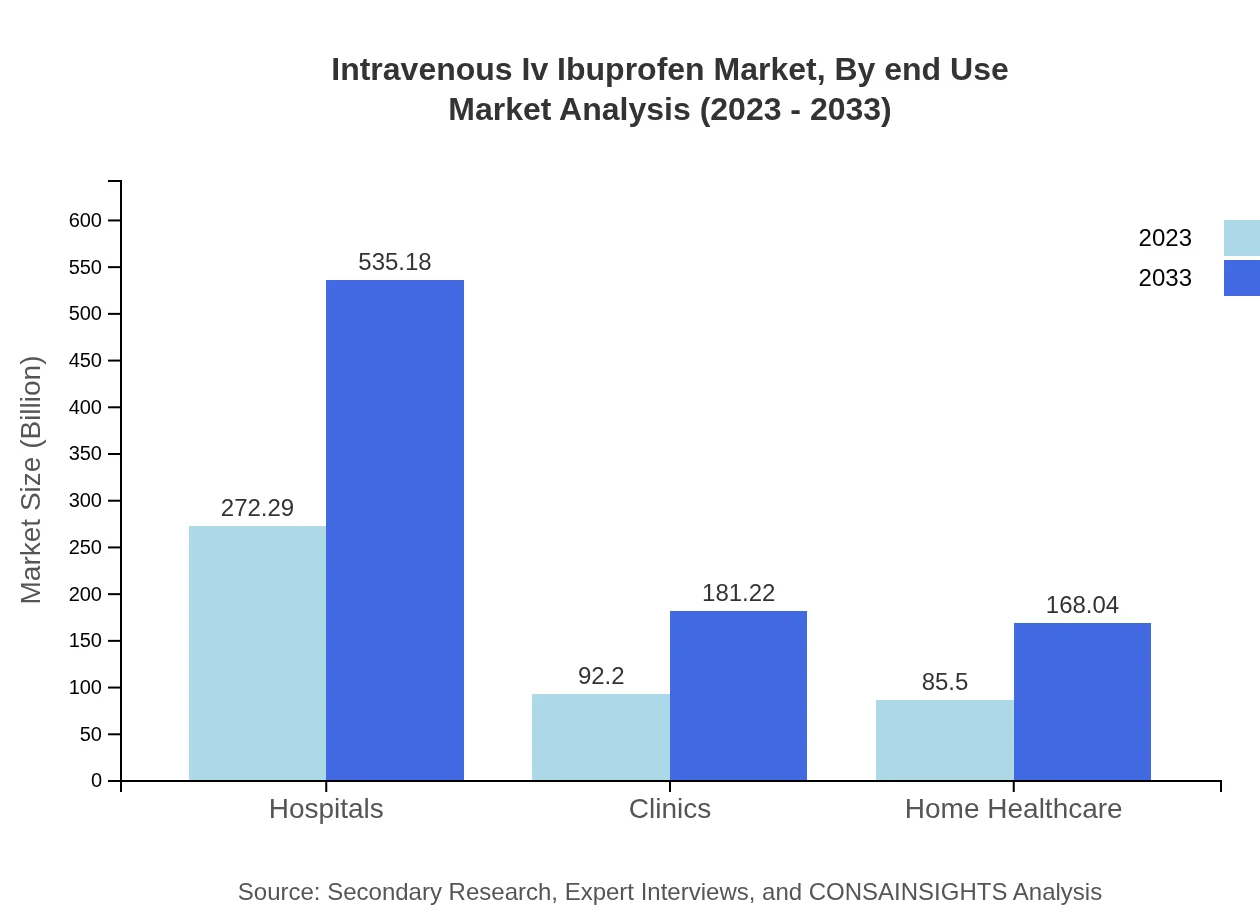
Intravenous Iv Ibuprofen Market Analysis By End Use
Hospitals remain the largest segment for IV Ibuprofen with 60.51% market share in 2023, attributed to intensive care and surgical recovery requirements. Clinics and home healthcare settings are also significant, reflecting a shift towards outpatient treatments and easy home administration procedures for pain management.
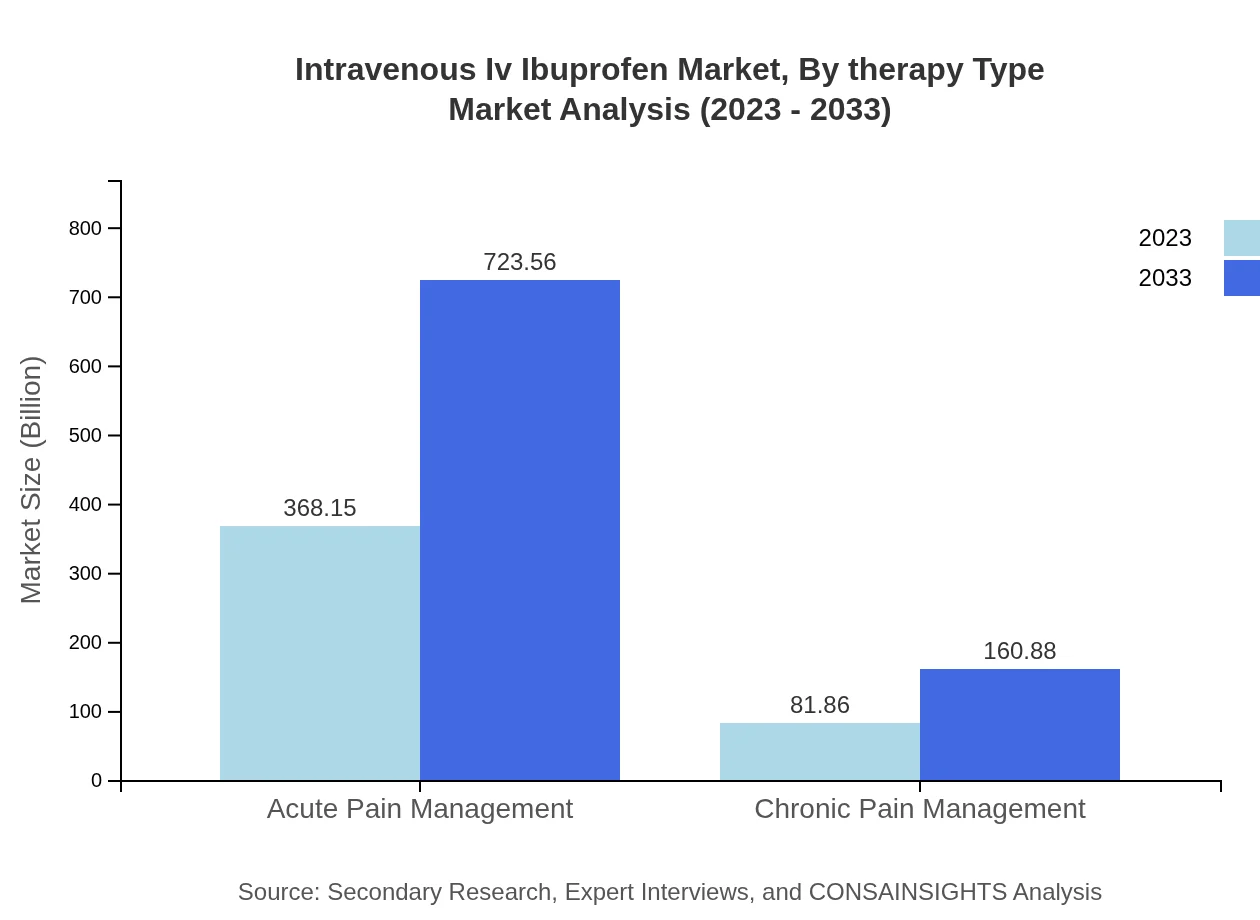
Intravenous Iv Ibuprofen Market Analysis By Therapy Type
The acute pain management segment is the largest, holding a prominent share of 81.81% in 2023, driven by a rise in post-operative pain treatments. Chronic pain management, while smaller at 18.19%, shows growing demand due to the increasing prevalence of long-term pain conditions.
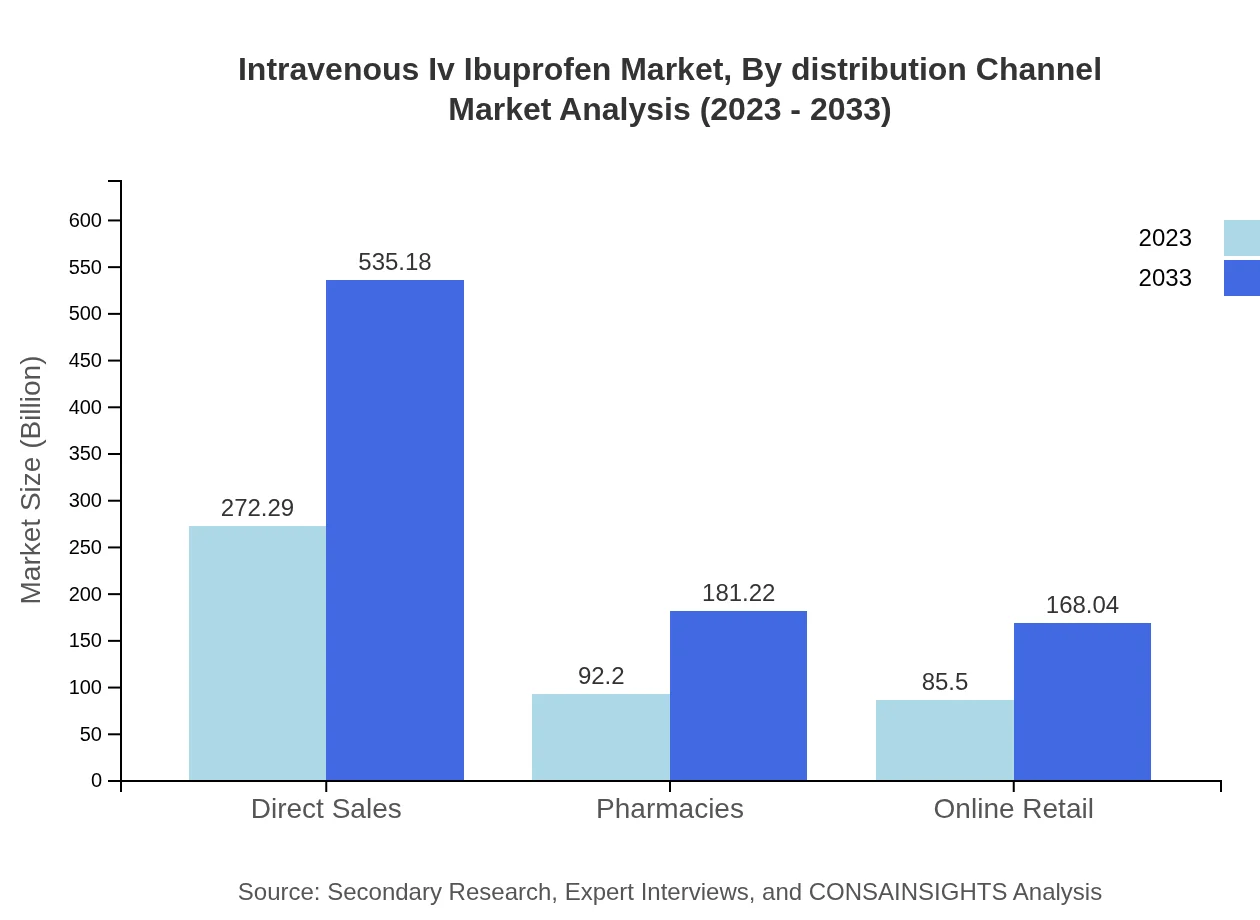
Intravenous Iv Ibuprofen Market Analysis By Distribution Channel
Direct sales dominate the distribution landscape, constituting 60.51% of the market share in 2023, benefiting from established relationships with healthcare providers. Pharmacies and online retail channels are emerging strongly as consumer preferences shift towards accessible pharmaceutical solutions.
Intravenous Iv Ibuprofen Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Intravenous Iv Ibuprofen Industry
ABC Pharmaceuticals:
One of the leading pharmaceutical companies focusing on innovative pain management solutions, contributing significantly to the IV Ibuprofen market with extensive R&D capabilities.XYZ Biotech:
A prominent player specializing in advanced drug formulations and targeted therapies, recognized for its high-quality IV Ibuprofen products tailored for hospital settings.MediCorp:
MediCorp is known for its expansive distribution network and commitment to providing affordable IV Ibuprofen options, making significant strides in patient access and outreach.We're grateful to work with incredible clients.









FAQs
What is the market size of intravenous Iv Ibuprofen?
The intravenous IV ibuprofen market is projected to reach approximately $450 million by 2033 with a compound annual growth rate (CAGR) of 6.8%. This growth reflects increasing utilization and acceptance in clinical settings for pain management.
What are the key market players or companies in this intravenous Iv Ibuprofen industry?
Key players in the intravenous IV ibuprofen market typically include pharmaceutical giants and specialized manufacturers focusing on novel drug delivery systems. These companies are critical in bringing innovative pain management solutions to healthcare providers.
What are the primary factors driving the growth in the intravenous Iv Ibuprofen industry?
The growth of the intravenous IV ibuprofen market is driven by increasing surgical procedures, growing demand for outpatient therapies, and the rising prevalence of chronic pain conditions requiring effective pain management alternatives.
Which region is the fastest Growing in the intravenous Iv Ibuprofen market?
The North American region is currently the fastest-growing market for intravenous IV ibuprofen, projected to grow from $170.91 million in 2023 to approximately $335.91 million by 2033, driven by advanced healthcare infrastructure and increasing patient needs.
Does ConsaInsights provide customized market report data for the intravenous Iv Ibuprofen industry?
Yes, ConsaInsights offers customized market reports tailored specifically for the intravenous IV ibuprofen industry. These reports can provide detailed insights based on client-specific requirements, ensuring relevant and actionable data.
What deliverables can I expect from this intravenous Iv Ibuprofen market research project?
Clients can expect detailed market analysis, segment breakdowns, forecasts, competitive landscape assessments, and actionable insights tailored to inform strategic decisions in the intravenous IV ibuprofen market segment.
What are the market trends of intravenous Iv Ibuprofen?
Key trends in the intravenous IV ibuprofen market include an increasing shift towards outpatient services, innovations in drug formulation, and greater emphasis on patient-centered pain management solutions as healthcare practices evolve.