Invitro Toxicology Toxicity Testing Market Report
Published Date: 31 January 2026 | Report Code: invitro-toxicology-toxicity-testing
Invitro Toxicology Toxicity Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a detailed analysis of the Invitro Toxicology Toxicity Testing market, offering insights into market size, growth forecasts, industry trends, and regional breakdowns from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
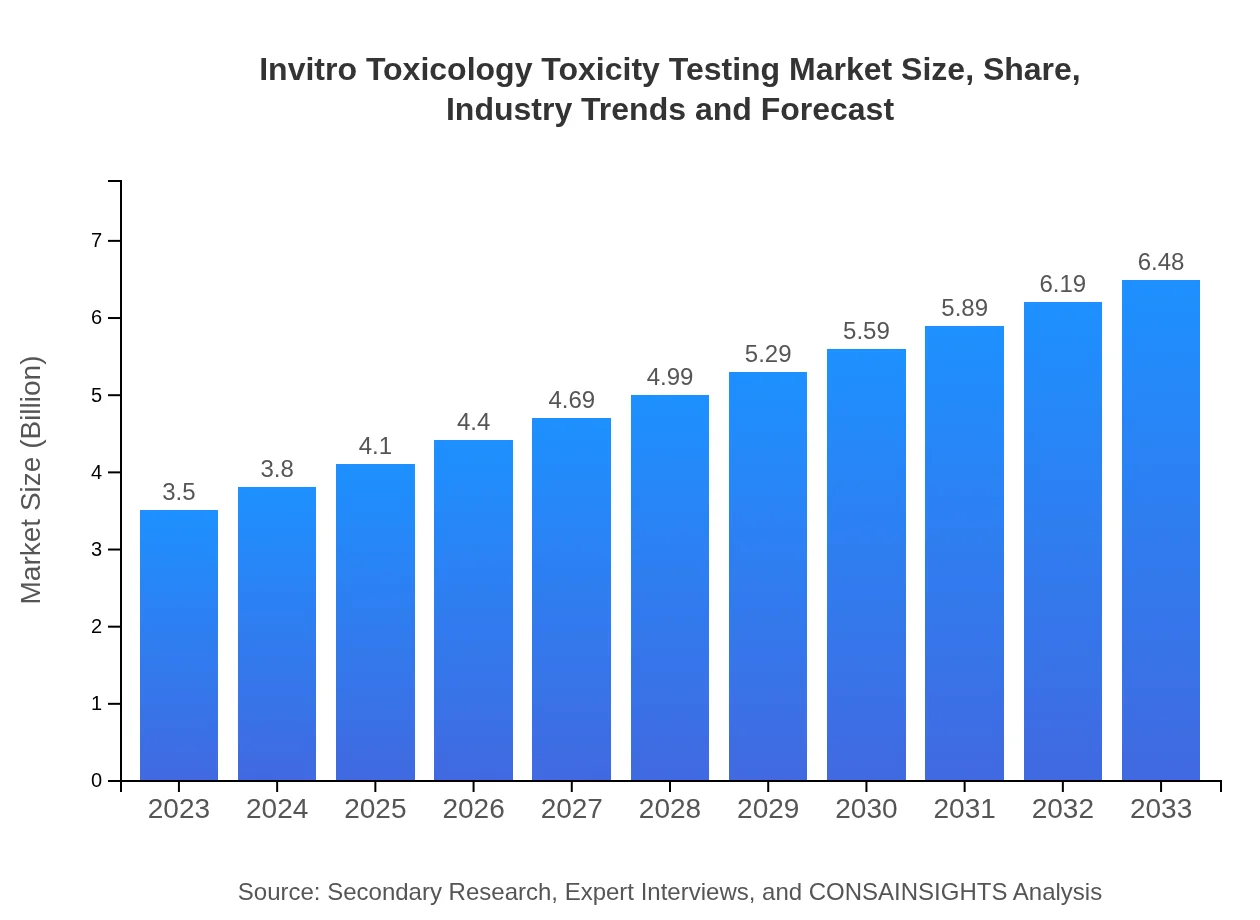
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $6.48 Billion |
| Top Companies | Charles River Laboratories, Eurofins Scientific, Sygnature Discovery, AccuGenomics, Boehringer Ingelheim |
| Last Modified Date | 31 January 2026 |
Invitro Toxicology Toxicity Testing Market Overview
Customize Invitro Toxicology Toxicity Testing Market Report market research report
- ✔ Get in-depth analysis of Invitro Toxicology Toxicity Testing market size, growth, and forecasts.
- ✔ Understand Invitro Toxicology Toxicity Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Invitro Toxicology Toxicity Testing
What is the Market Size & CAGR of Invitro Toxicology Toxicity Testing market in 2023?
Invitro Toxicology Toxicity Testing Industry Analysis
Invitro Toxicology Toxicity Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Invitro Toxicology Toxicity Testing Market Analysis Report by Region
Europe Invitro Toxicology Toxicity Testing Market Report:
The European Invitro Toxicology Toxicity Testing market is projected to increase from $1.00 billion in 2023 to $1.85 billion by 2033. Factors such as strong governmental policies promoting alternative testing and the expansion of biopharmaceutical sectors contribute to this increase.Asia Pacific Invitro Toxicology Toxicity Testing Market Report:
In the Asia Pacific region, the Invitro Toxicology Toxicity Testing market was valued at $0.66 billion in 2023, projected to grow to $1.22 billion by 2033. The growth is attributed to rising healthcare expenditure, increased research activities, and a shift towards advanced testing modalities in Japan, China, and India.North America Invitro Toxicology Toxicity Testing Market Report:
North America remains the largest market, accounting for $1.33 billion in 2023, projected to grow to $2.47 billion by 2033. This growth is driven by a robust pharmaceutical industry, stringent regulatory frameworks, and increased funding for toxicology research.South America Invitro Toxicology Toxicity Testing Market Report:
The South American market for Invitro Toxicology Toxicity Testing is valued at $0.12 billion in 2023 and is expected to enhance, reaching $0.23 billion by 2033. The growth is fueled by rising investments in pharmaceutical research, coupled with an increasing regulatory focus on safety testing.Middle East & Africa Invitro Toxicology Toxicity Testing Market Report:
In the Middle East and Africa, the Invitro Toxicology Toxicity Testing market is anticipated to grow from $0.39 billion in 2023 to $0.72 billion by 2033, aided by growing awareness of medical advancements and increasing funding for toxicological studies.Tell us your focus area and get a customized research report.
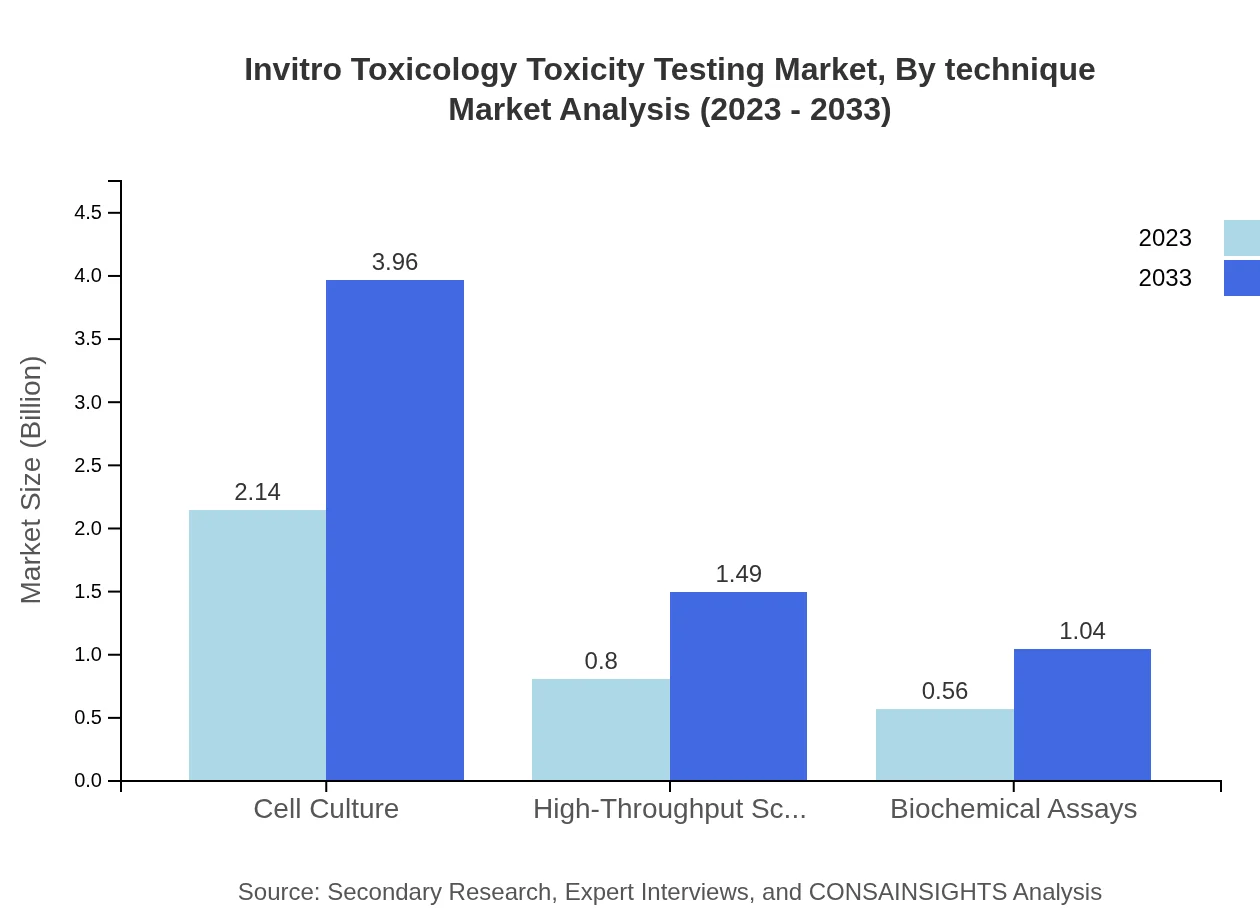
Invitro Toxicology Toxicity Testing Market Analysis By Technique
The by-technique segment includes biochemical assays, cell culture, and high-throughput screening as dominant techniques driving market revenue. Each plays a critical role in comprehensive toxicity assessments based on the specific requirements of products and regulatory landscapes.
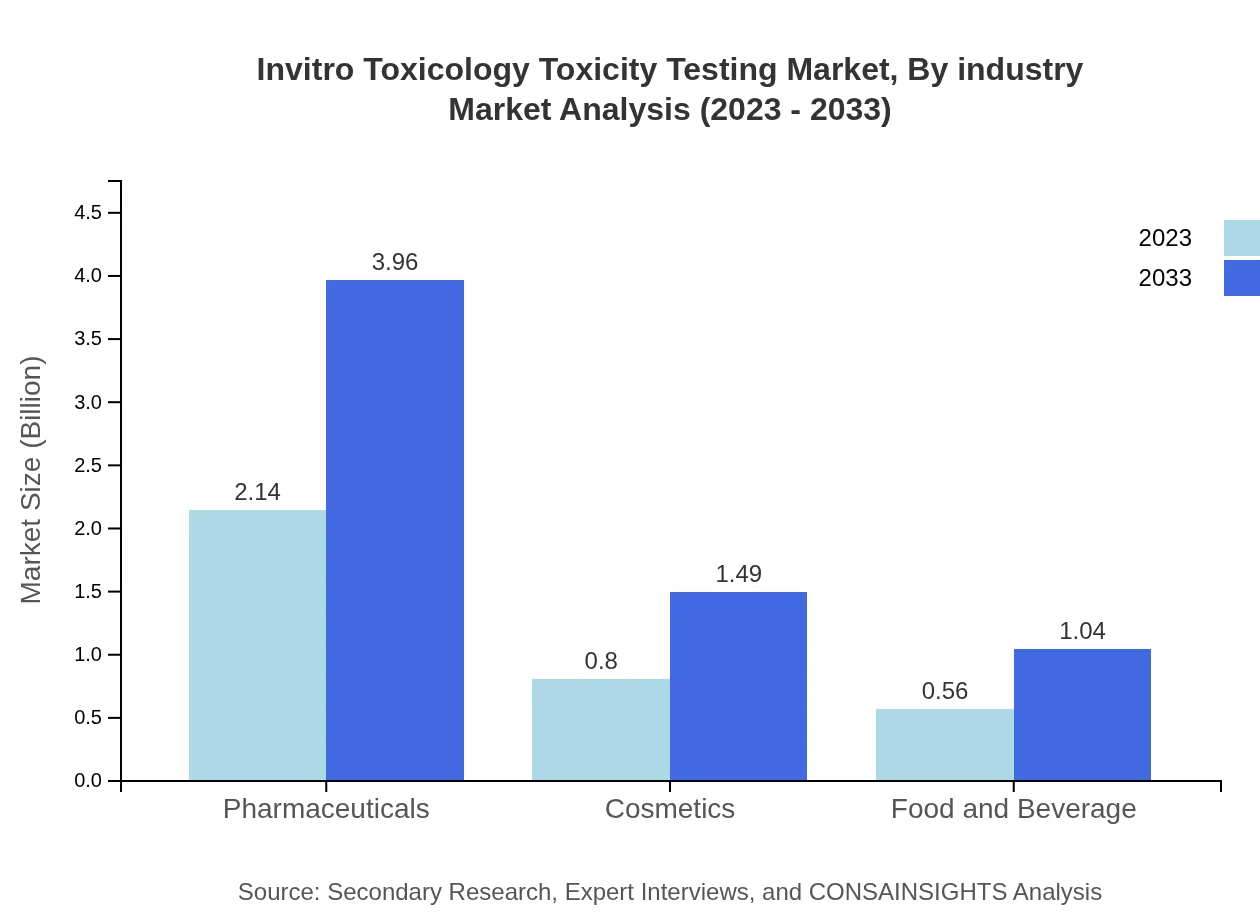
Invitro Toxicology Toxicity Testing Market Analysis By Industry
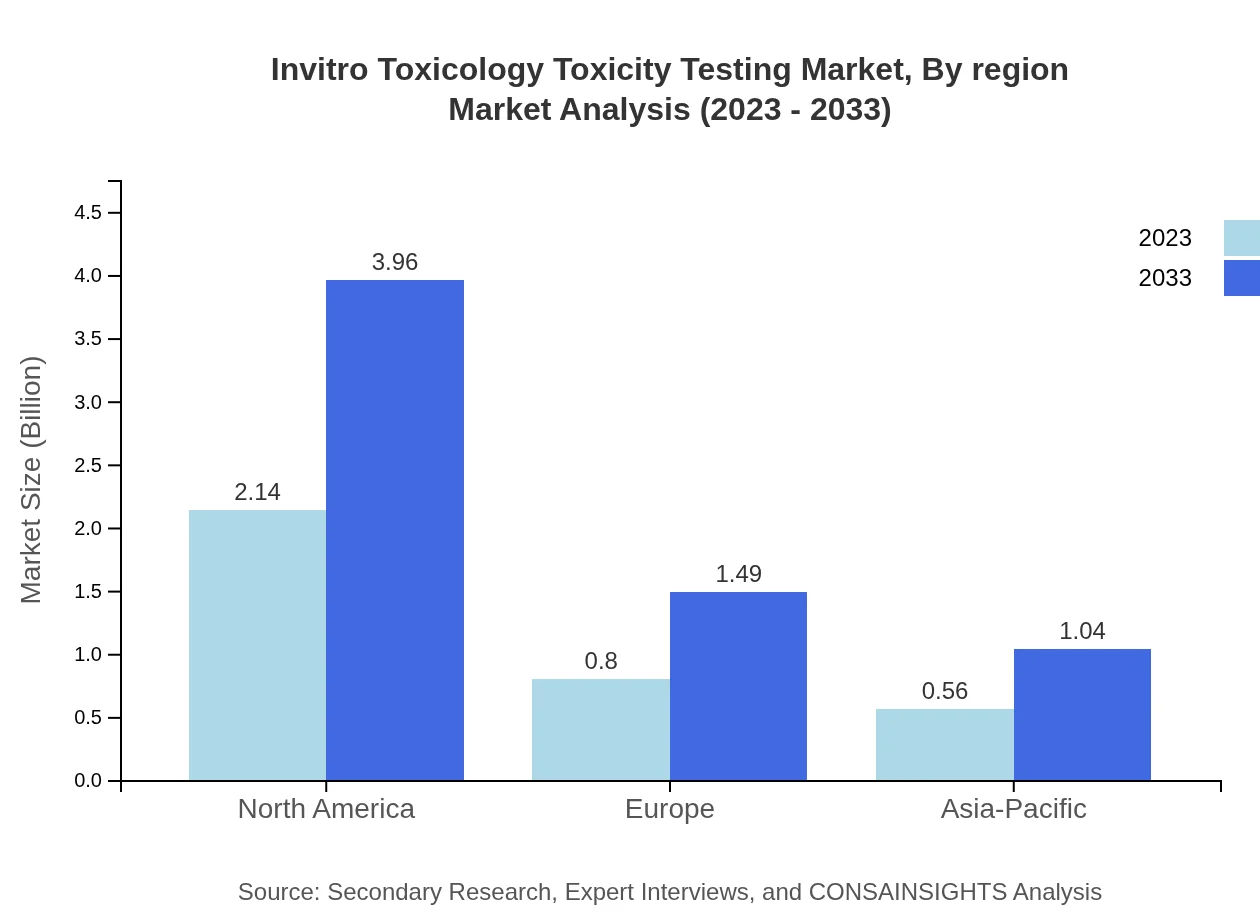
The pharmaceutical industry remains the largest contributor, holding a market size of $2.14 billion in 2023, growing to $3.96 billion by 2033. The cosmetics segment follows, estimated at $0.80 billion in 2023, rising to $1.49 billion. The food and beverage sector is also emerging with market growth anticipated from $0.56 billion to $1.04 billion.
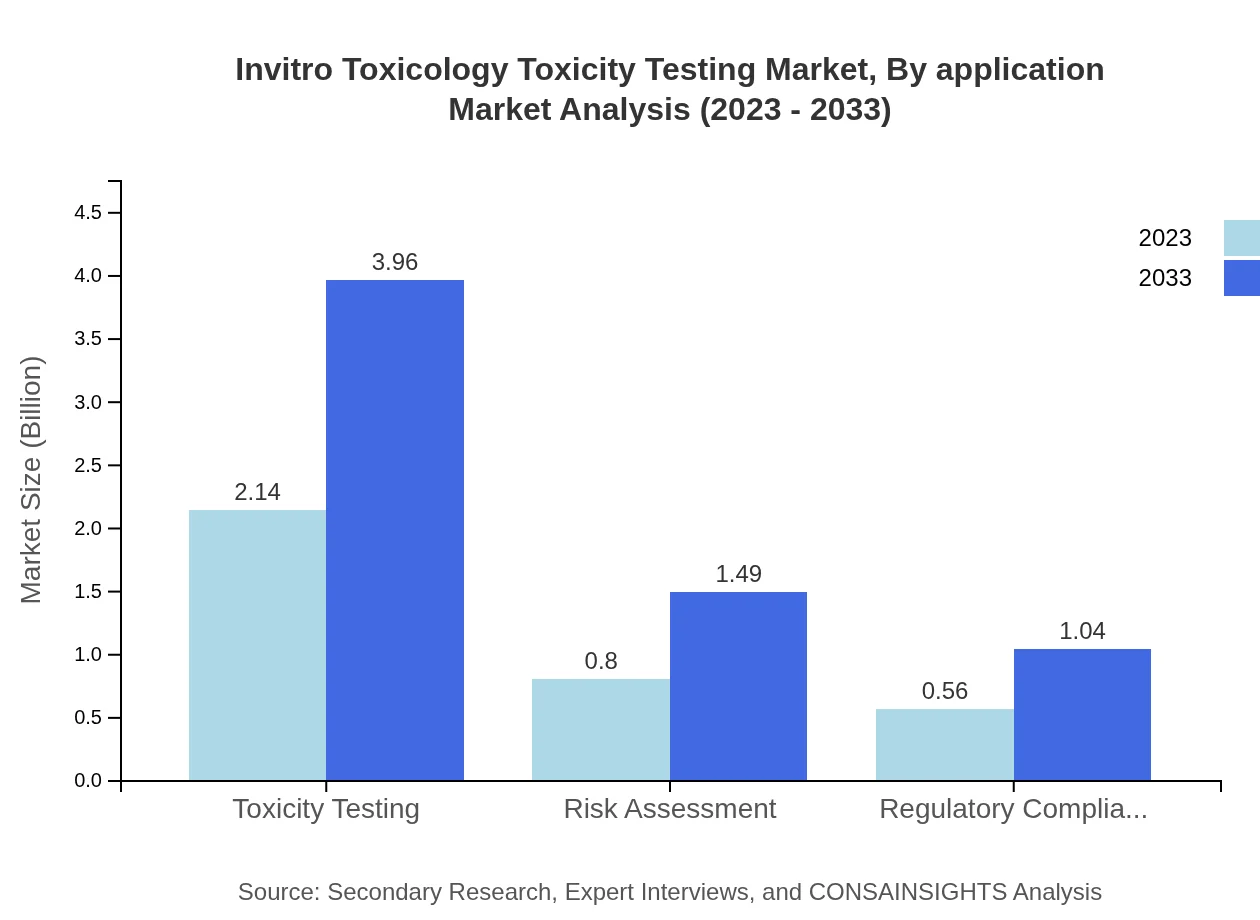
Invitro Toxicology Toxicity Testing Market Analysis By Application
Applications in toxicity testing, risk assessment, and regulatory compliance are vital for ensuring product safety across various industries. The market for toxicity testing is valued at $2.14 billion in 2023, expected to reach $3.96 billion by 2033.
Invitro Toxicology Toxicity Testing Market Analysis By Region
Regional analyses show disparities in market growth with North America commanding a significant share, while Asia Pacific shows potential for rapid growth due to increasing investments in research and development.
Invitro Toxicology Toxicity Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Invitro Toxicology Toxicity Testing Industry
Charles River Laboratories:
A leading provider of preclinical and clinical laboratory services for the pharmaceutical, medical device, and biotechnology industries, known for innovative toxicology testing.Eurofins Scientific:
A key player in the laboratory services sector, offering a comprehensive range of toxicity testing solutions tailored to regulatory standards.Sygnature Discovery:
Specializes in drug discovery and development, providing extensive in vitro testing capabilities across therapeutic areas.AccuGenomics:
Offers innovative biotechnology services, including in vitro toxicology screening to support drug development and safety assessment.Boehringer Ingelheim:
Globally renowned pharmaceutical company contributing to the development of alternative testing methodologies in toxicology.We're grateful to work with incredible clients.









FAQs
What is the market size of invitro Toxicology Toxicity Testing?
The invitro toxicology toxicity testing market is valued at approximately $3.5 billion in 2023, with an expected growth at a CAGR of 6.2%, indicating a robust demand for alternative testing methods across various industries.
What are the key market players or companies in this invitro Toxicology Toxicity Testing industry?
Key players in the invitro toxicology toxicity testing market include companies specializing in pharmaceutical development, safety assessment, and contract research organizations, playing crucial roles in shaping industry standards and technological advancements.
What are the primary factors driving the growth in the invitro Toxicology Toxicity Testing industry?
Factors driving growth include the increasing regulatory pressures on animal testing, advancements in cell culture technologies, and a shift towards more efficient and ethical testing methodologies that favor invitro solutions over traditional approaches.
Which region is the fastest Growing in the invitro Toxicology Toxicity Testing?
North America is the fastest-growing region in the invitro toxicology toxicity testing market, projected to grow from $1.33 billion in 2023 to approximately $2.47 billion by 2033, driven by robust research initiatives and funding.
Does ConsaInsights provide customized market report data for the invitro Toxicology Toxicity Testing industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the invitro toxicology toxicity testing industry, ensuring comprehensive insights that cater to unique business requirements.
What deliverables can I expect from this invitro Toxicology Toxicity Testing market research project?
Deliverables typically include detailed market analysis reports, trends forecasts, segmented data insights, competitive landscape assessments, and strategic recommendations tailored for stakeholders in the invitro toxicology sector.
What are the market trends of invitro Toxicology Toxicity Testing?
Current trends in the invitro toxicology toxicity testing market include increasing automation in laboratories, integration of AI in analysis processes, and a focus on developing more complex 3D cell models for more accurate results.