Iv Bags Market Report
Published Date: 31 January 2026 | Report Code: iv-bags
Iv Bags Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the IV Bags market from 2023 to 2033, including insights into market size, industry trends, regional performance, and critical segmentation analysis.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
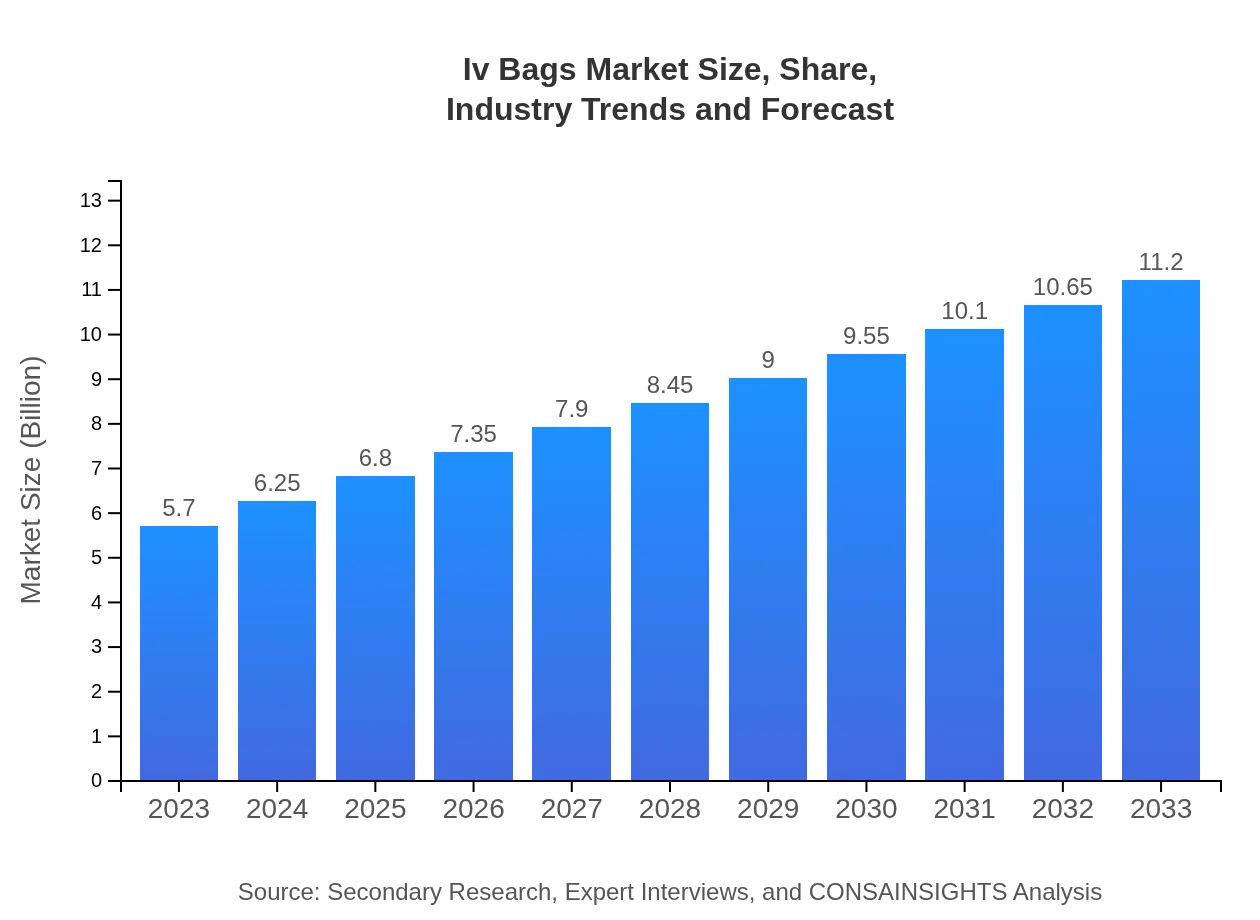
| 2023 Market Size | $5.70 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $11.20 Billion |
| Top Companies | Baxter International Inc., B. Braun Melsungen AG, ICU Medical, Inc. |
| Last Modified Date | 31 January 2026 |
IV Bags Market Overview
Customize Iv Bags Market Report market research report
- ✔ Get in-depth analysis of Iv Bags market size, growth, and forecasts.
- ✔ Understand Iv Bags's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Iv Bags
What is the Market Size & CAGR of IV Bags market in 2023?
IV Bags Industry Analysis
IV Bags Market Segmentation and Scope
Tell us your focus area and get a customized research report.
IV Bags Market Analysis Report by Region
Europe Iv Bags Market Report:
Europe's IV Bags market size is anticipated to grow from $1.66 billion in 2023 to $3.25 billion by 2033. Strong healthcare regulations and a high emphasis on patient safety drive the expansion of the IV Bags market in the region, particularly in the UK, Germany, and France.Asia Pacific Iv Bags Market Report:
The Asia Pacific region is anticipated to witness significant growth, with the market size expected to grow from $1.19 billion in 2023 to $2.35 billion by 2033. This growth can be attributed to increasing healthcare expenditures and a rising frequency of surgeries in countries like India and China.North America Iv Bags Market Report:
North America continues to dominate the IV Bags market, with the size projected to reach $3.64 billion in 2033 from $1.85 billion in 2023 due to advanced healthcare facilities, high treatment rates, and innovations in medical technology.South America Iv Bags Market Report:
In South America, the IV Bags market size is projected to increase from $0.32 billion in 2023 to $0.64 billion in 2033. Factors include improving healthcare infrastructure and increasing awareness about healthcare in Brazil and Argentina.Middle East & Africa Iv Bags Market Report:
The Middle East and Africa market size for IV Bags is projected to increase from $0.67 billion in 2023 to $1.32 billion in 2033. The growth is driven by improving healthcare quality and increasing investments in the medical sector, especially in GCC countries.Tell us your focus area and get a customized research report.
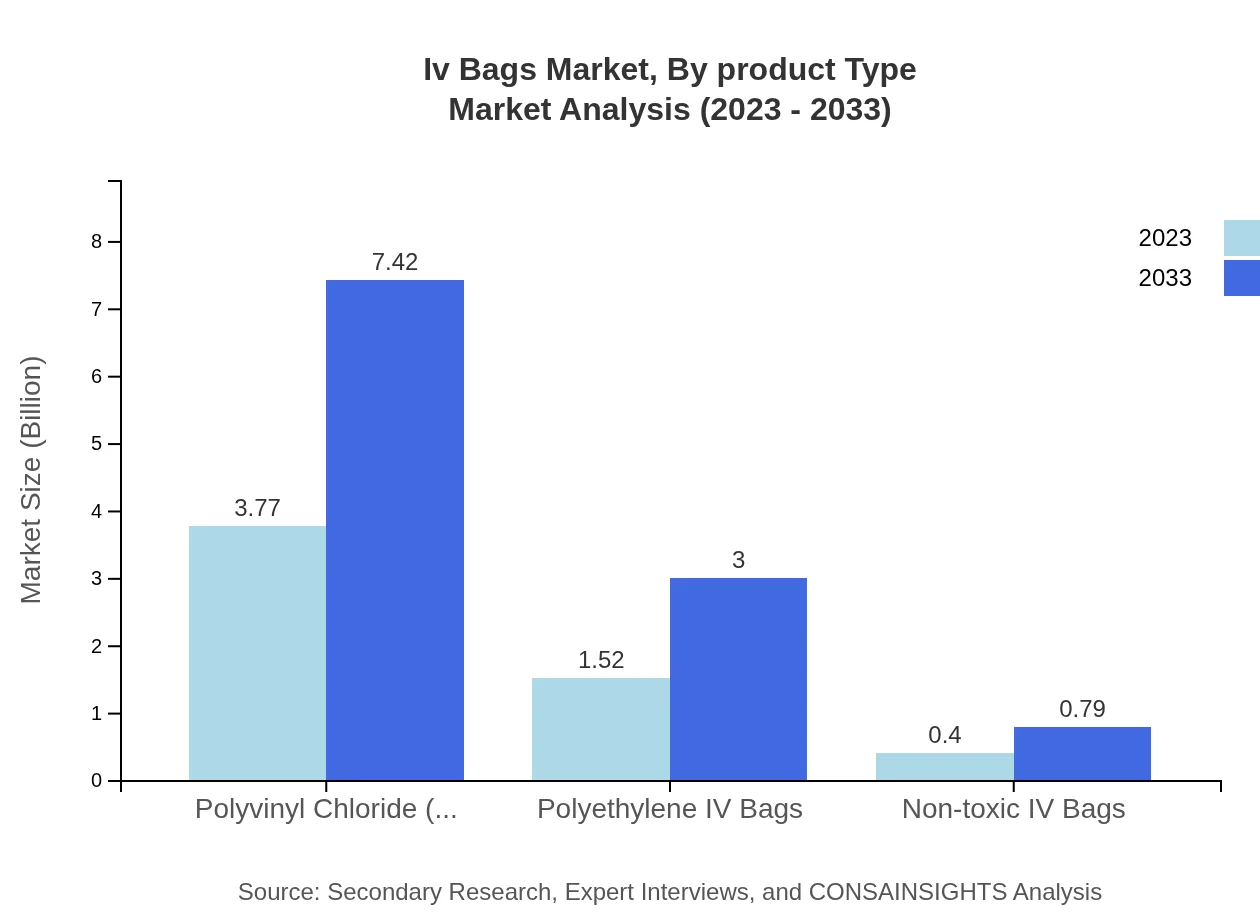
Iv Bags Market Analysis By Product Type
The IV Bags market is primarily segmented into Polyvinyl Chloride (PVC) and Polyethylene IV Bags. PVC IV Bags are expected to maintain a leading market share of 66.19% in 2023 due to their flexible properties and cost-effectiveness. Polyethylene IV Bags, holding 26.74% market share, are gaining traction due to their lower plasticizer content and non-toxic nature, appealing to healthcare providers aiming to reduce patient exposure to harmful chemicals.
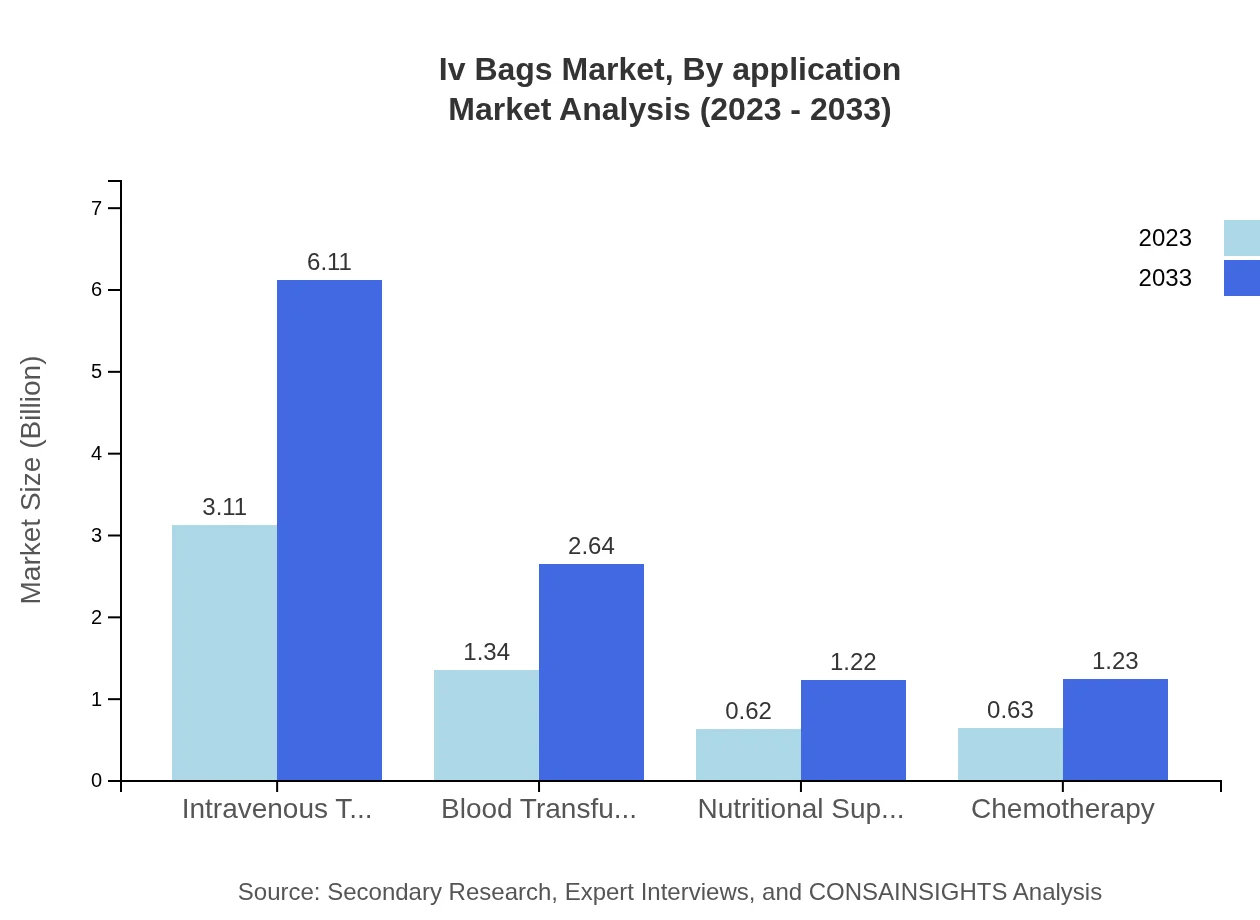
Iv Bags Market Analysis By Application
Major application segments within the IV Bags market include intravenous therapy, blood transfusion, and nutritional support. Intravenous therapy leads the market with a significant share of 54.58% and is projected to grow with increasing hospitalizations and outpatient procedures. Blood transfusion and nutritional support segments exhibit growing demand, particularly in oncology and chronic disease management.
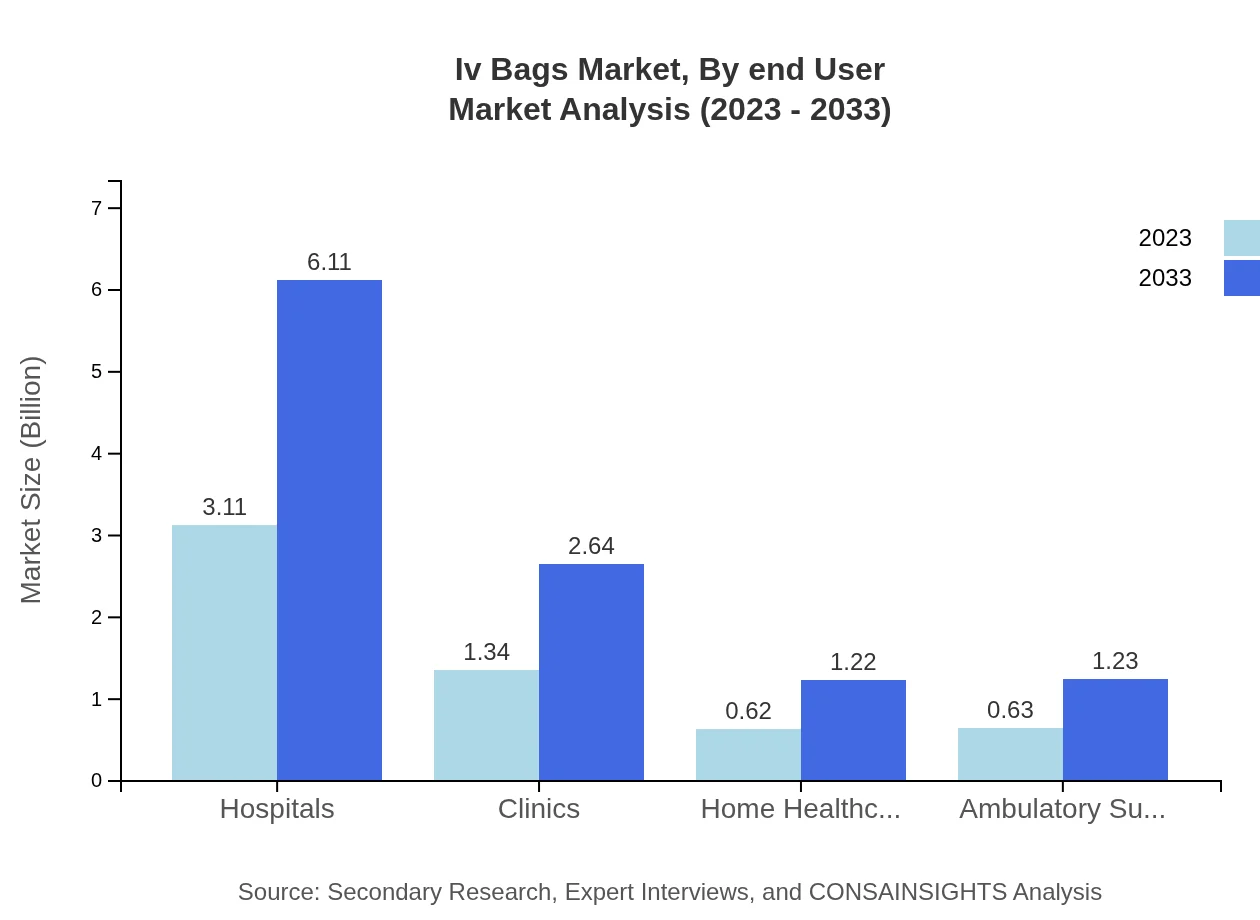
Iv Bags Market Analysis By End User
Hospitals dominate the IV Bags market, with a share of 54.58% in 2023, driven by high patient volumes and a need for safe medication delivery. Clinics and ambulatory surgery centers are also substantial contributors, alongside a rising share from home healthcare, which is projected to increase significantly as patient-centric care models gain popularity.
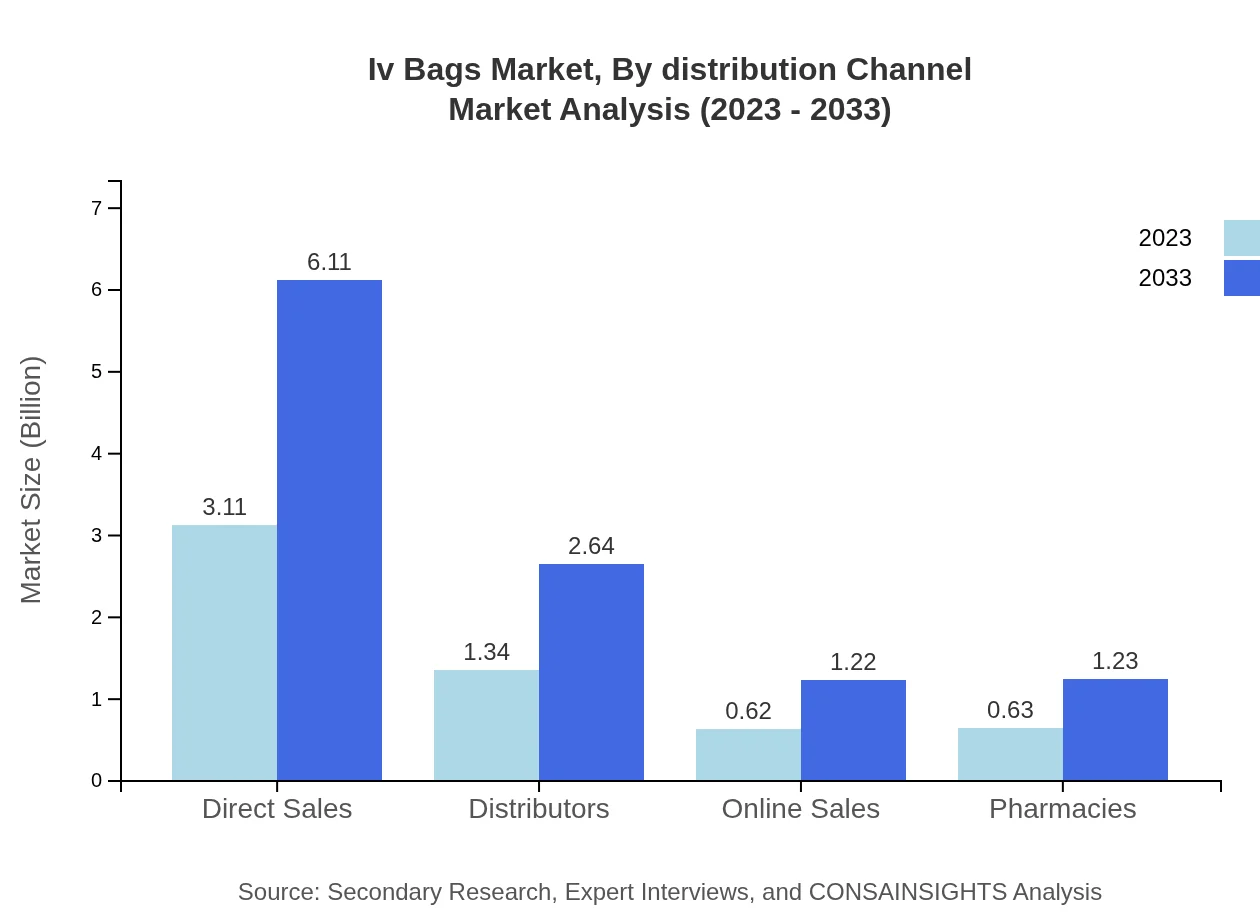
Iv Bags Market Analysis By Distribution Channel
Distribution channels for IV Bags include direct sales, distributors, online sales, and pharmacies. Direct sales projected to account for 54.58% market share, followed by distributors at 23.57%. The increasing trend of online sales in healthcare products signifies a shift in consumer purchasing behavior, projected to grow as e-commerce becomes a more prevalent distribution method.
IV Bags Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in IV Bags Industry
Baxter International Inc.:
Baxter is a global leader in IV solutions and the largest manufacturer of IV Bags, providing innovative products to hospitals worldwide.B. Braun Melsungen AG:
B. Braun is known for its extensive range of IV therapy products, including both standard and advanced IV Bags designed with safety features.ICU Medical, Inc.:
ICU Medical is recognized for its infusion therapy devices and innovative IV solutions, enhancing patient safety and quality of care.We're grateful to work with incredible clients.









FAQs
What is the market size of IV Bags?
The global IV bags market is currently valued at $5.7 billion, with a projected CAGR of 6.8%. This growth indicates a significant demand for intravenous fluid delivery systems, which are vital in various healthcare settings.
What are the key market players or companies in the IV bags industry?
Key players in the IV bags industry include B. Braun Melsungen AG, Baxter International Inc., and Fresenius Kabi AG, among others. These companies drive innovation, enhancing the product quality and expanding their market presence.
What are the primary factors driving the growth in the IV bags industry?
The growth in the IV bags market is driven by the rising prevalence of chronic diseases, increased surgical procedures, and the growing aging population, demanding efficient intravenous therapies and solutions.
Which region is the fastest Growing in the IV bags market?
The Asia Pacific region is the fastest-growing in the IV bags market. By 2033, its market size is expected to double from $1.19 billion in 2023 to $2.35 billion, showcasing rapid healthcare development.
Does ConsaInsights provide customized market report data for the IV bags industry?
Yes, ConsaInsights offers customized market report data tailored specifically to the IV bags industry. These reports can be designed to meet unique client requirements, allowing deeper insights into market trends.
What deliverables can I expect from this IV bags market research project?
Deliverables from the IV bags market research project typically include market analysis reports, competitor analysis, growth forecasts, and regional market insights, providing comprehensive coverage of the industry landscape.
What are the market trends of IV bags?
Current trends in the IV bags market include increasing adoption of non-toxic IV bags, advancements in materials like PVC and polyethylene, and a rise in online sales channels, reflecting evolving consumer preferences.