Lab Automation In Clinical Diagnostics Market Report
Published Date: 22 January 2026 | Report Code: lab-automation-in-clinical-diagnostics
Lab Automation In Clinical Diagnostics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Lab Automation In Clinical Diagnostics market, focusing on current trends, market size, and forecasts from 2023 to 2033. It includes insights into various segments, regional performance, and key industry players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
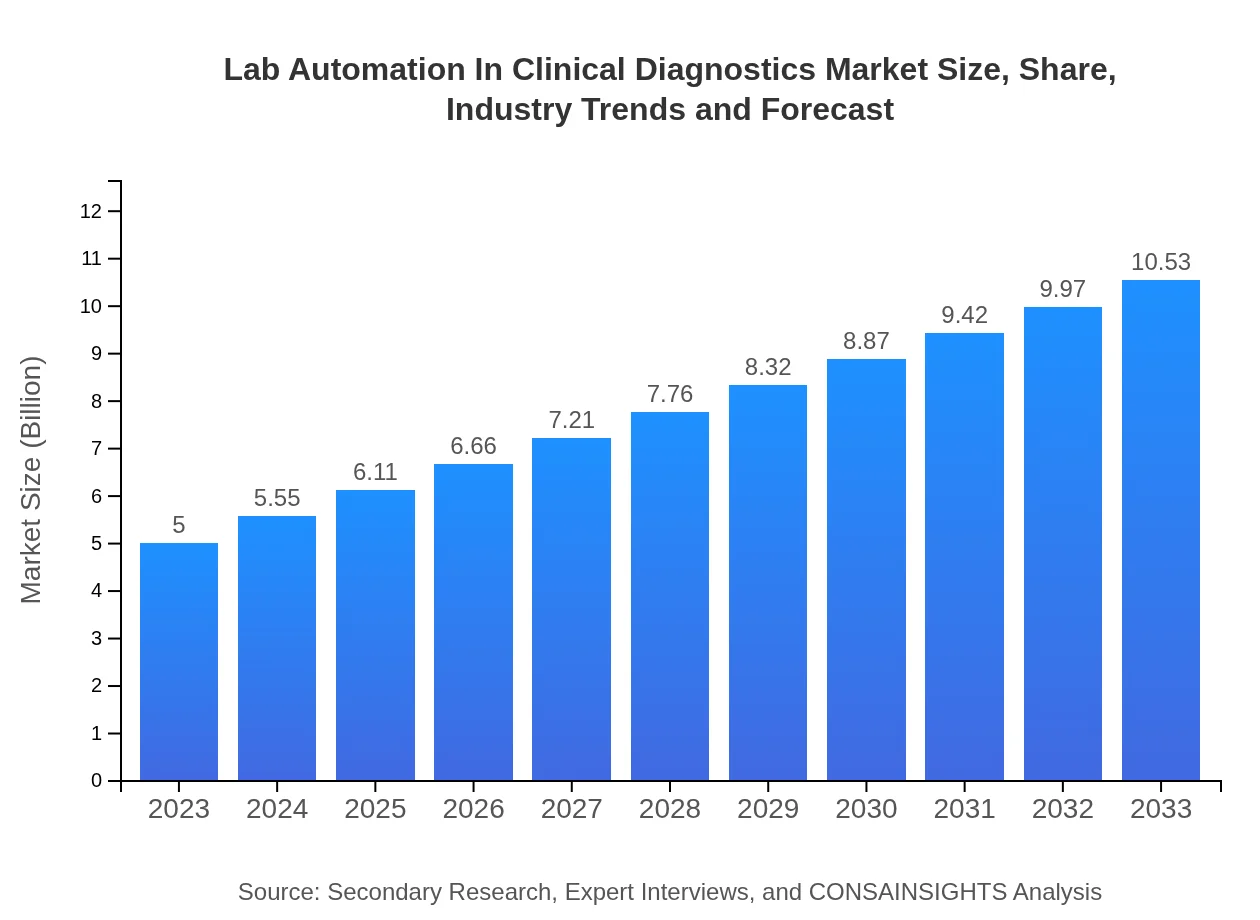
| 2023 Market Size | $5.00 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $10.53 Billion |
| Top Companies | Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific, Roche Diagnostics, Beckman Coulter |
| Last Modified Date | 22 January 2026 |
Lab Automation In Clinical Diagnostics Market Overview
Customize Lab Automation In Clinical Diagnostics Market Report market research report
- ✔ Get in-depth analysis of Lab Automation In Clinical Diagnostics market size, growth, and forecasts.
- ✔ Understand Lab Automation In Clinical Diagnostics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Lab Automation In Clinical Diagnostics
What is the Market Size & CAGR of Lab Automation In Clinical Diagnostics market in 2023?
Lab Automation In Clinical Diagnostics Industry Analysis
Lab Automation In Clinical Diagnostics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Lab Automation In Clinical Diagnostics Market Analysis Report by Region
Europe Lab Automation In Clinical Diagnostics Market Report:
The European market is anticipated to rise from $1.39 billion in 2023 to $2.92 billion by 2033. Factors such as stringent regulations and the need for higher efficiency in laboratories fuel market growth.Asia Pacific Lab Automation In Clinical Diagnostics Market Report:
The Asia Pacific region reflects significant growth potential in the Lab Automation In Clinical Diagnostics market, rising from $1.00 billion in 2023 to $2.11 billion by 2033. Increasing healthcare expenditure and a growing number of laboratories are driving this expansion.North America Lab Automation In Clinical Diagnostics Market Report:
North America is the leading market, with a size expected to grow from $1.74 billion in 2023 to $3.65 billion by 2033. High adoption rates of advanced technologies and robust healthcare infrastructure are key factors contributing to this growth.South America Lab Automation In Clinical Diagnostics Market Report:
In South America, the market is projected to grow from $0.39 billion in 2023 to $0.82 billion by 2033. The region is experiencing growth due to an increase in public and private healthcare investments focused on improving diagnostic capabilities.Middle East & Africa Lab Automation In Clinical Diagnostics Market Report:
In the Middle East and Africa, the market is estimated to grow from $0.49 billion in 2023 to $1.03 billion by 2033, driven by improving healthcare access and enhancement of laboratory services.Tell us your focus area and get a customized research report.
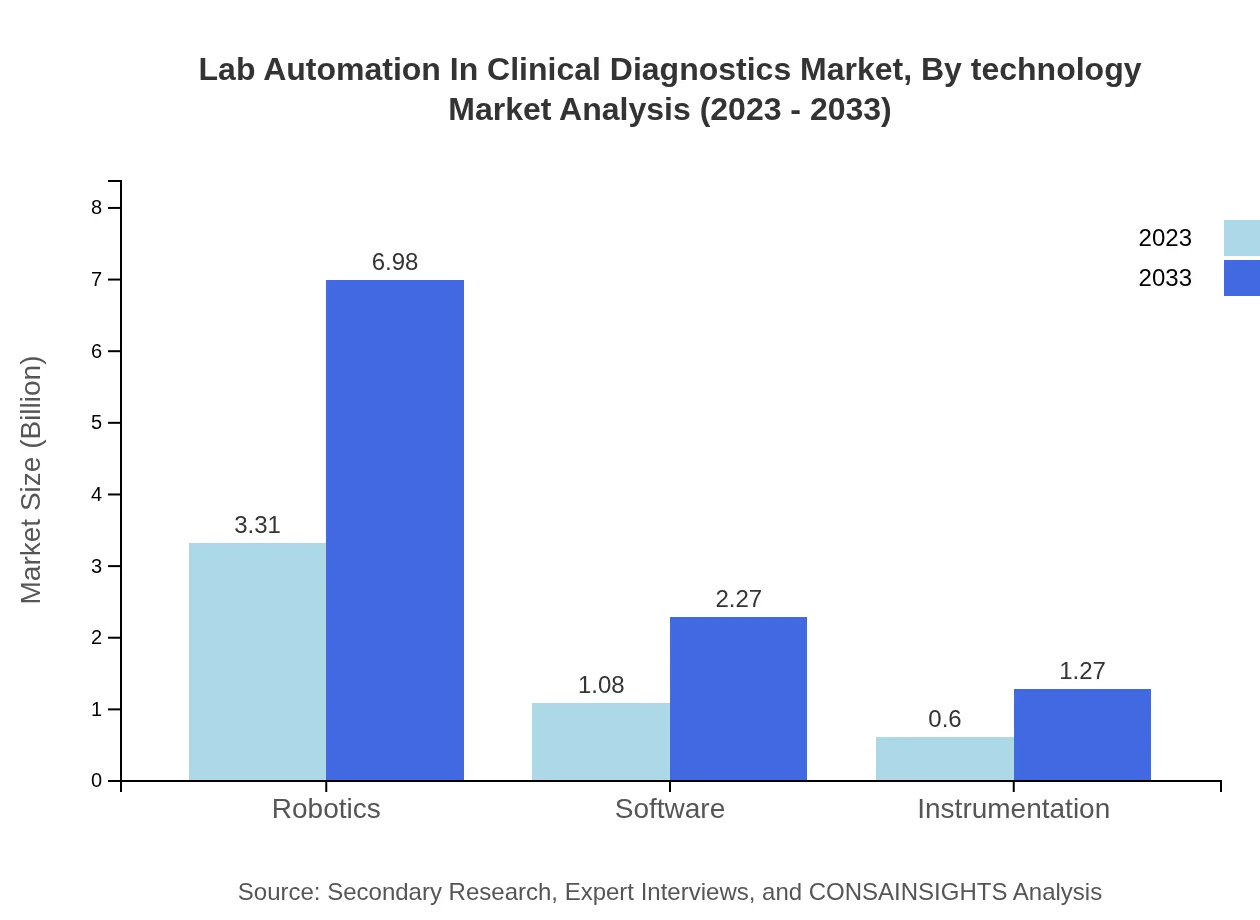
Lab Automation In Clinical Diagnostics Market Analysis By Technology
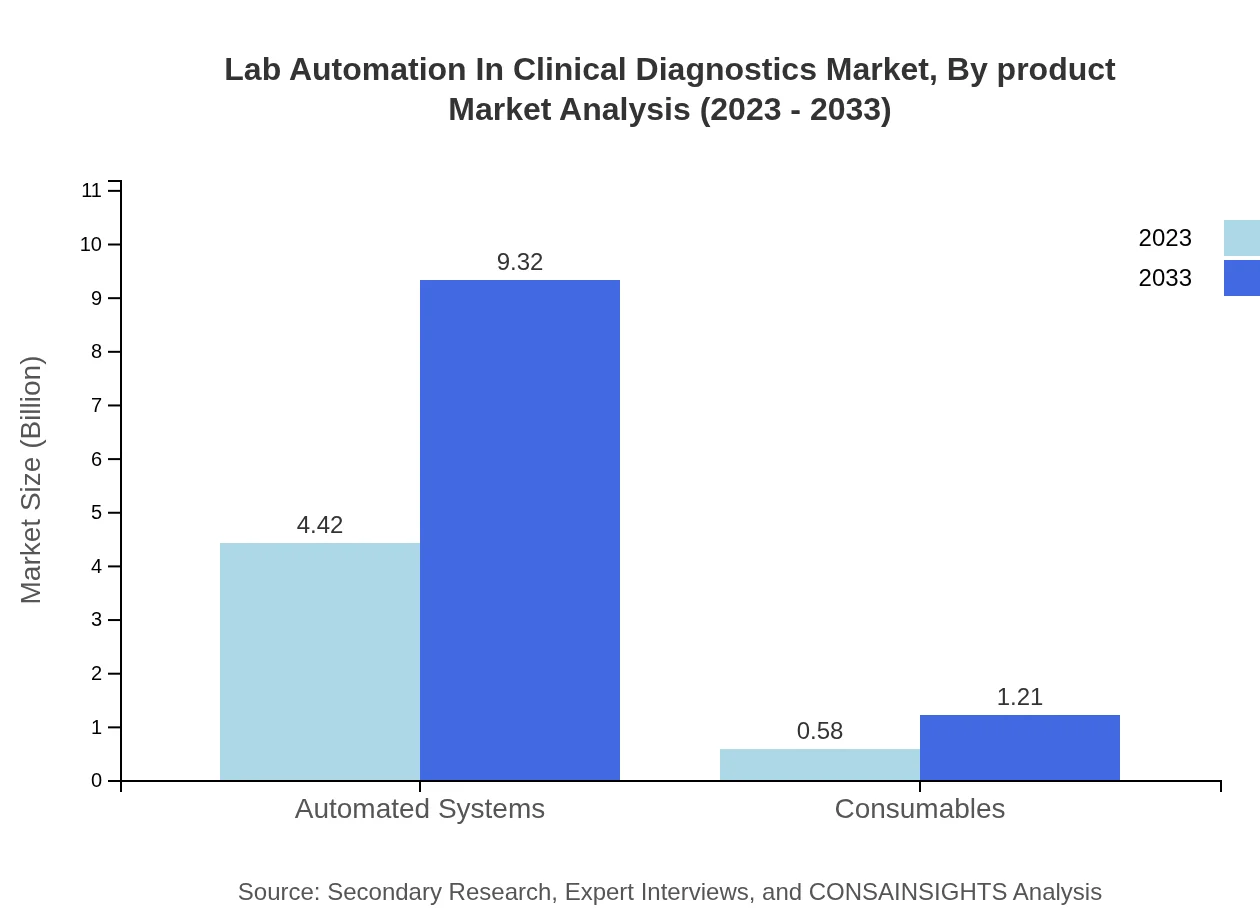
The Lab Automation in Clinical Diagnostics market, segmented by technology, showcases substantial growth in automated systems. The market size for automated systems is projected to increase from $4.42 billion in 2023 to $9.32 billion by 2033, maintaining a market share of 88.5% across the forecast period. Consumables and software follow, with consumables expected to expand from $0.58 billion to $1.21 billion.
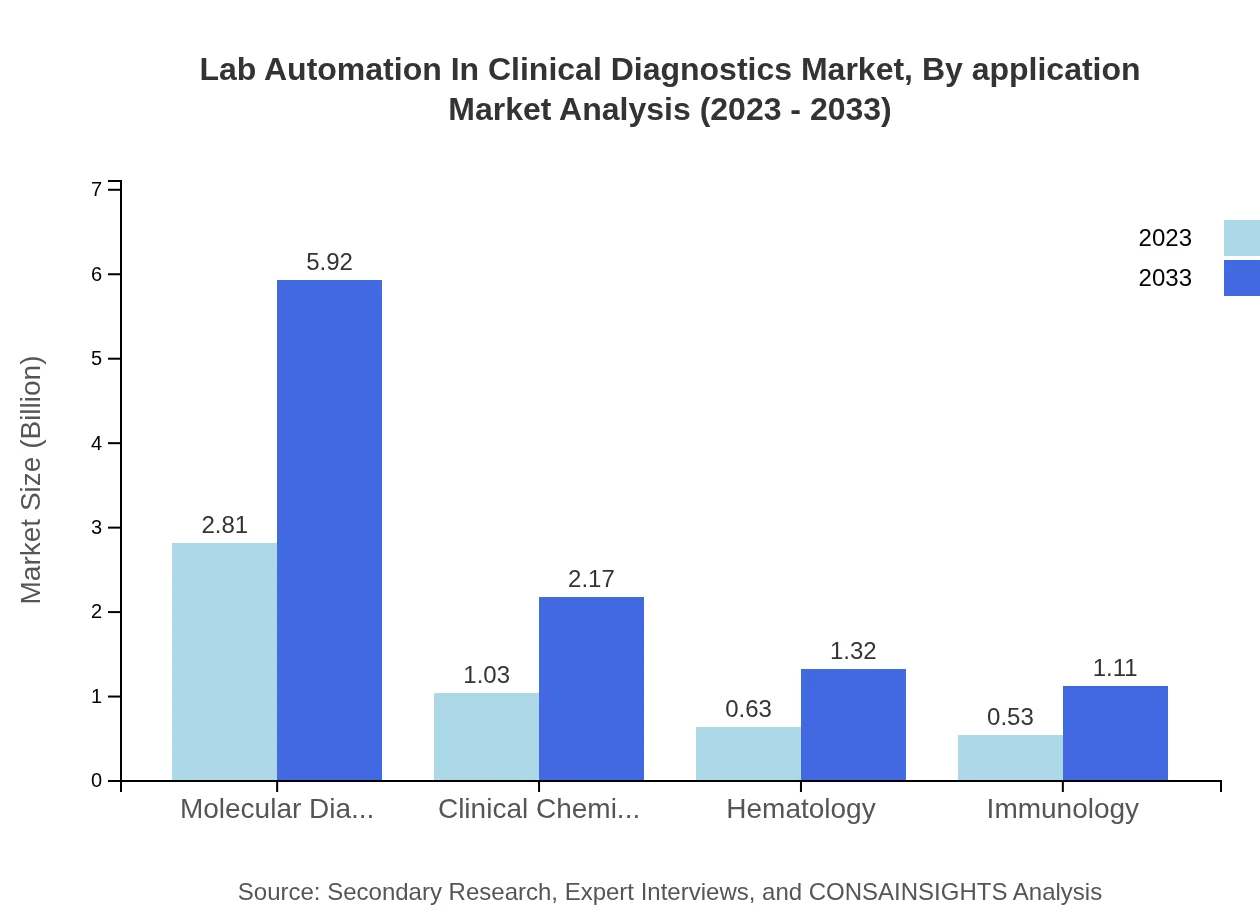
Lab Automation In Clinical Diagnostics Market Analysis By Application
When considering applications, hospitals dominate with a market size that is expected to grow from $3.31 billion in 2023 to $6.98 billion by 2033. Diagnostic laboratories and research institutes play significant roles as well, with expected resizing of $1.08 billion to $2.27 billion and $0.60 billion to $1.27 billion, respectively.
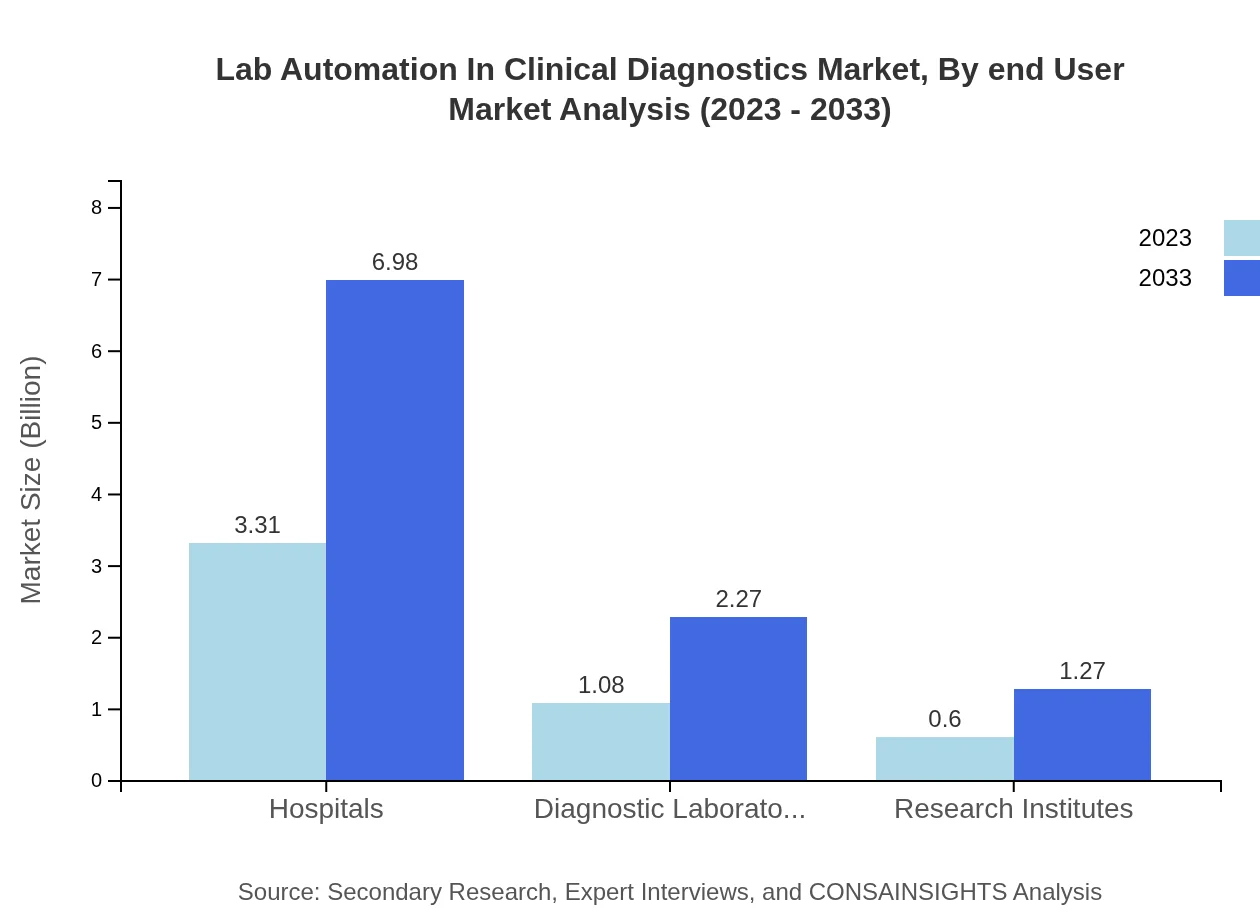
Lab Automation In Clinical Diagnostics Market Analysis By End User
The analysis of end-users reveals hospitals, diagnostic laboratories, and research institutes are the primary consumers of lab automation technologies. The hospital sector accounts for a largest market share, while diagnostic labs and institutes steadily increase their adoption.
Lab Automation In Clinical Diagnostics Market Analysis By Product
In terms of products, automated systems are leading, primarily due to their critical role in expediting workflows. Consumables follow closely, indicating a growing demand for ongoing support and integration with automation solutions.
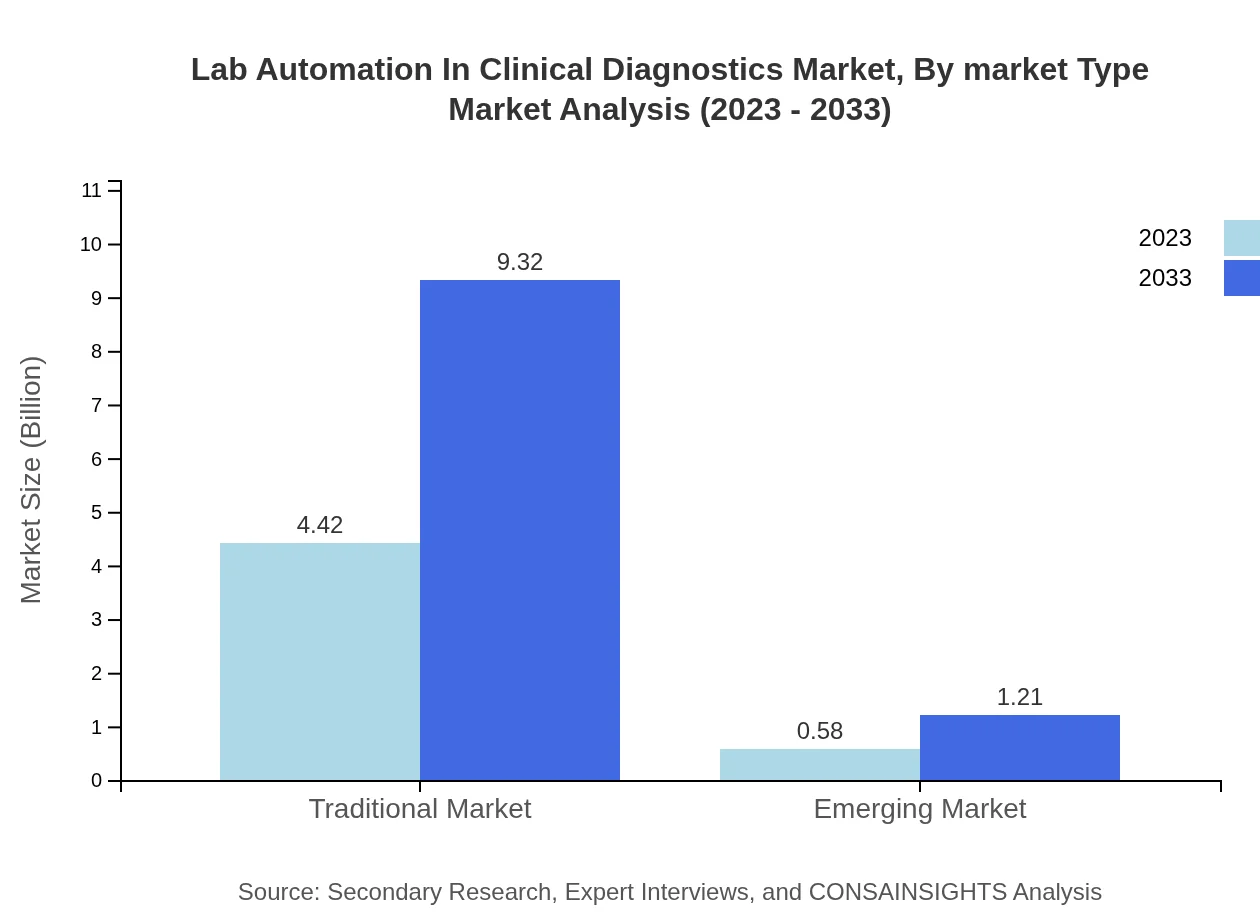
Lab Automation In Clinical Diagnostics Market Analysis By Market Type
The market is divided into traditional and emerging segments, with the traditional market anticipated to maintain a dominant position, supported by established practices and proven technologies. The emerging market is also seeing a positive trend as labs integrate newer technologies.
Lab Automation In Clinical Diagnostics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Lab Automation In Clinical Diagnostics Industry
Abbott Laboratories:
Abbott is known for its innovative diagnostic solutions and extensive portfolio in lab automation, focusing on enhancing laboratory efficiency through advanced technology.Siemens Healthineers:
Siemens Healthineers offers a wide range of laboratory automation solutions, contributing significantly to improving patient outcomes with reliable diagnostics.Thermo Fisher Scientific:
Thermo Fisher Scientific specializes in laboratory instruments and automation solutions that help laboratories achieve high efficiency and accuracy in diagnostics.Roche Diagnostics:
Roche is a leader in diagnostics and pursues advancements in automation technology to optimize laboratory workflows and enhance diagnostic reliability.Beckman Coulter:
Beckman Coulter provides a range of solutions designed to improve workflow and productivity in clinical laboratories, focusing on high-throughput automation and innovative software.We're grateful to work with incredible clients.









FAQs
What is the market size of lab automation in clinical diagnostics?
The lab automation in clinical diagnostics market is expected to grow from $5 billion in 2023 to an estimated size in 2033, with a CAGR of 7.5%. This growth is attributed to the rising demand for efficient diagnostic solutions.
What are the key market players or companies in this lab automation in clinical diagnostics industry?
Key players in the lab automation market include major companies focused on technological advancements and product innovations. They aim to enhance diagnostic capabilities and improve efficiency in laboratories, ultimately positioning themselves as market leaders.
What are the primary factors driving the growth in the lab automation in clinical diagnostics industry?
Growth in the lab automation market is driven by increasing demand for accurate and faster diagnostic testing, technological advancements in automation processes, and the rising prevalence of chronic diseases needing effective diagnostic solutions.
Which region is the fastest Growing in the lab automation in clinical diagnostics?
The fastest-growing regions in the lab automation market include North America and Europe. For instance, North America is projected to grow from $1.74 billion in 2023 to $3.65 billion in 2033, showcasing substantial market growth.
Does ConsaInsights provide customized market report data for the lab automation in clinical diagnostics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the lab automation industry. Clients can request reports that focus on particular segments, geographical areas, or emerging trends for more insightful analysis.
What deliverables can I expect from this lab automation in clinical diagnostics market research project?
The deliverables include comprehensive market analysis reports, detailed segment data, growth trend projections, and insights on key competitors in the lab automation field, enabling informed decision-making and strategy development.
What are the market trends of lab automation in clinical diagnostics?
Notable market trends in lab automation include a shift towards integration of AI technologies for diagnostic accuracy, increased demand for automated systems in hospitals, and a greater focus on molecular diagnostics due to their efficiency and precision.