Lateral Flow Assays Market Report
Published Date: 31 January 2026 | Report Code: lateral-flow-assays
Lateral Flow Assays Market Size, Share, Industry Trends and Forecast to 2033
This report explores the global Lateral Flow Assays market, providing comprehensive insights and data forecasts from 2023 to 2033. It includes analyses of market size, growth rates, industry dynamics, regional performance, technology trends, and competitive landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
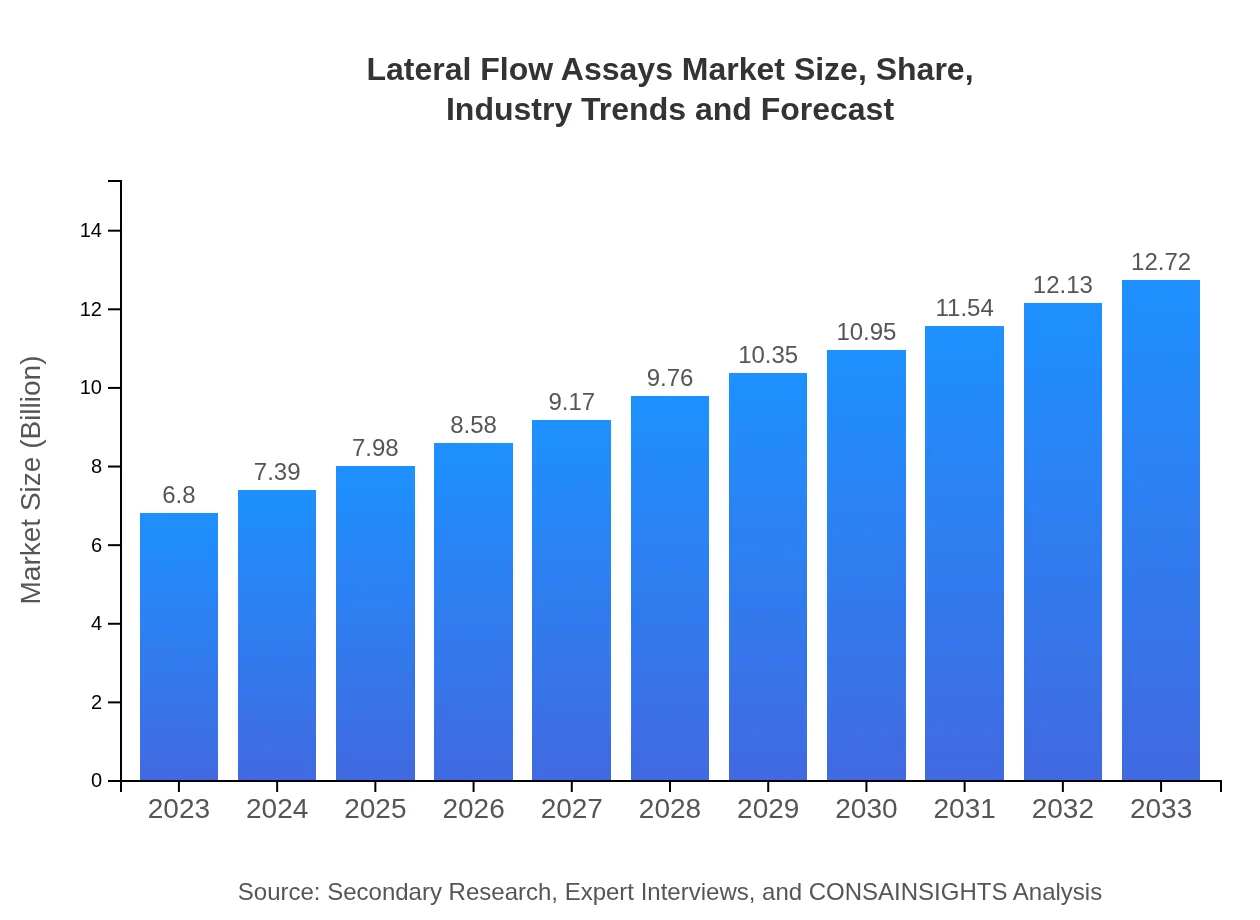
| 2023 Market Size | $6.80 Billion |
| CAGR (2023-2033) | 6.3% |
| 2033 Market Size | $12.72 Billion |
| Top Companies | Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, Abingdon Health |
| Last Modified Date | 31 January 2026 |
Lateral Flow Assays Market Overview
Customize Lateral Flow Assays Market Report market research report
- ✔ Get in-depth analysis of Lateral Flow Assays market size, growth, and forecasts.
- ✔ Understand Lateral Flow Assays's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Lateral Flow Assays
What is the Market Size & CAGR of Lateral Flow Assays market in 2023?
Lateral Flow Assays Industry Analysis
Lateral Flow Assays Market Segmentation and Scope
Tell us your focus area and get a customized research report.
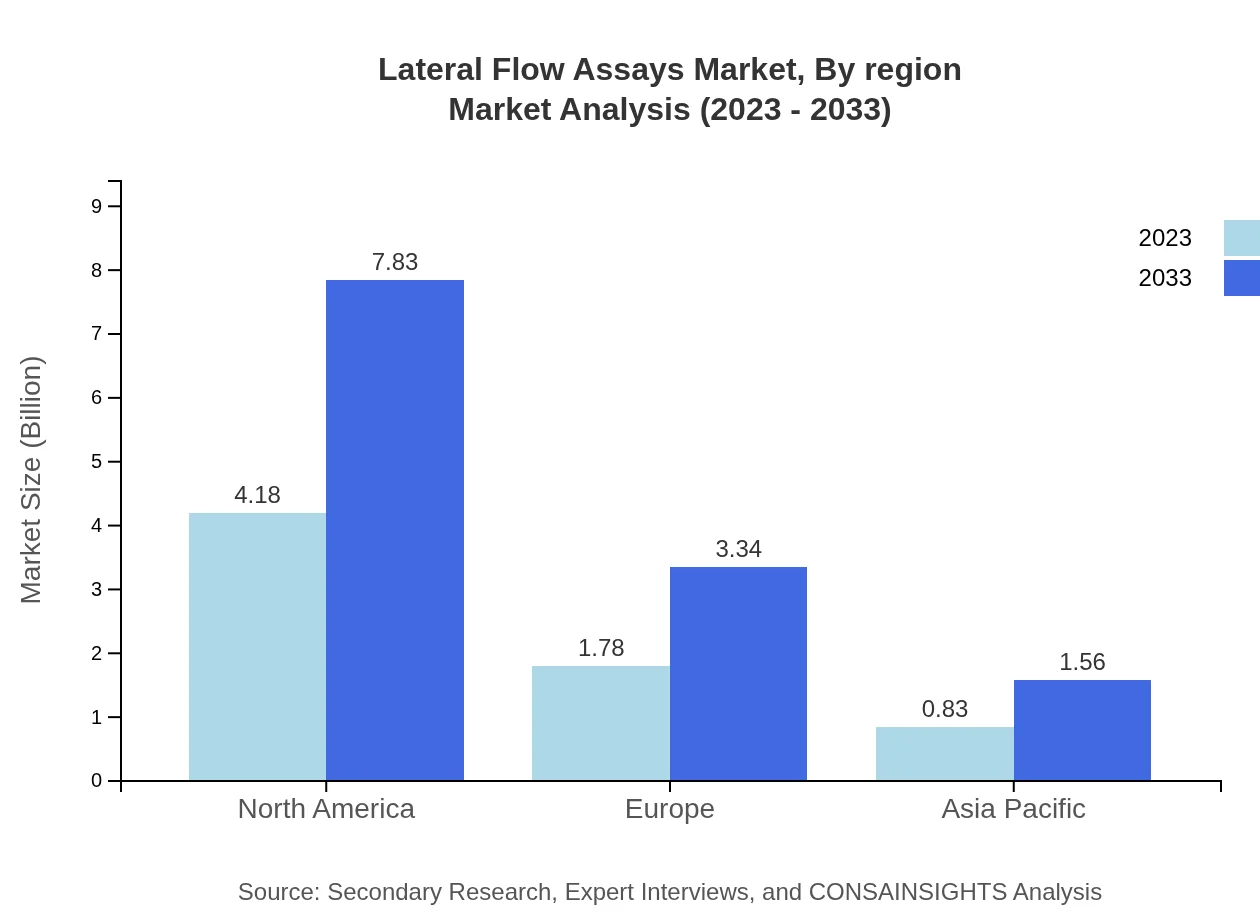
Lateral Flow Assays Market Analysis Report by Region
Europe Lateral Flow Assays Market Report:
With a market size of $2.44 billion in 2023, Europe is projected to grow to $4.57 billion by 2033, supported by advancements in healthcare infrastructures and regulatory support for rapid testing solutions.Asia Pacific Lateral Flow Assays Market Report:
In the Asia Pacific region, the lateral flow assays market has been growing steadily, with a valuation of $1.13 billion in 2023 and a projected rise to $2.12 billion by 2033, driven by the increasing healthcare expenditures and investments in diagnostic technologies.North America Lateral Flow Assays Market Report:
North America remains the largest market for lateral flow assays, valued at $2.26 billion in 2023 and anticipated to grow to $4.22 billion by 2033, fueled by a high prevalence of infectious diseases and a strong demand for point-of-care diagnostics.South America Lateral Flow Assays Market Report:
The South American market for lateral flow assays is expected to witness moderate growth, starting at $0.65 billion in 2023 and reaching $1.23 billion by 2033, as public health initiatives increase the emphasis on rapid disease detection.Middle East & Africa Lateral Flow Assays Market Report:
The Middle East and Africa market for lateral flow assays, valued at $0.31 billion in 2023, is expected to experience gradual growth, reaching $0.59 billion by 2033, as healthcare access improves in various regions.Tell us your focus area and get a customized research report.
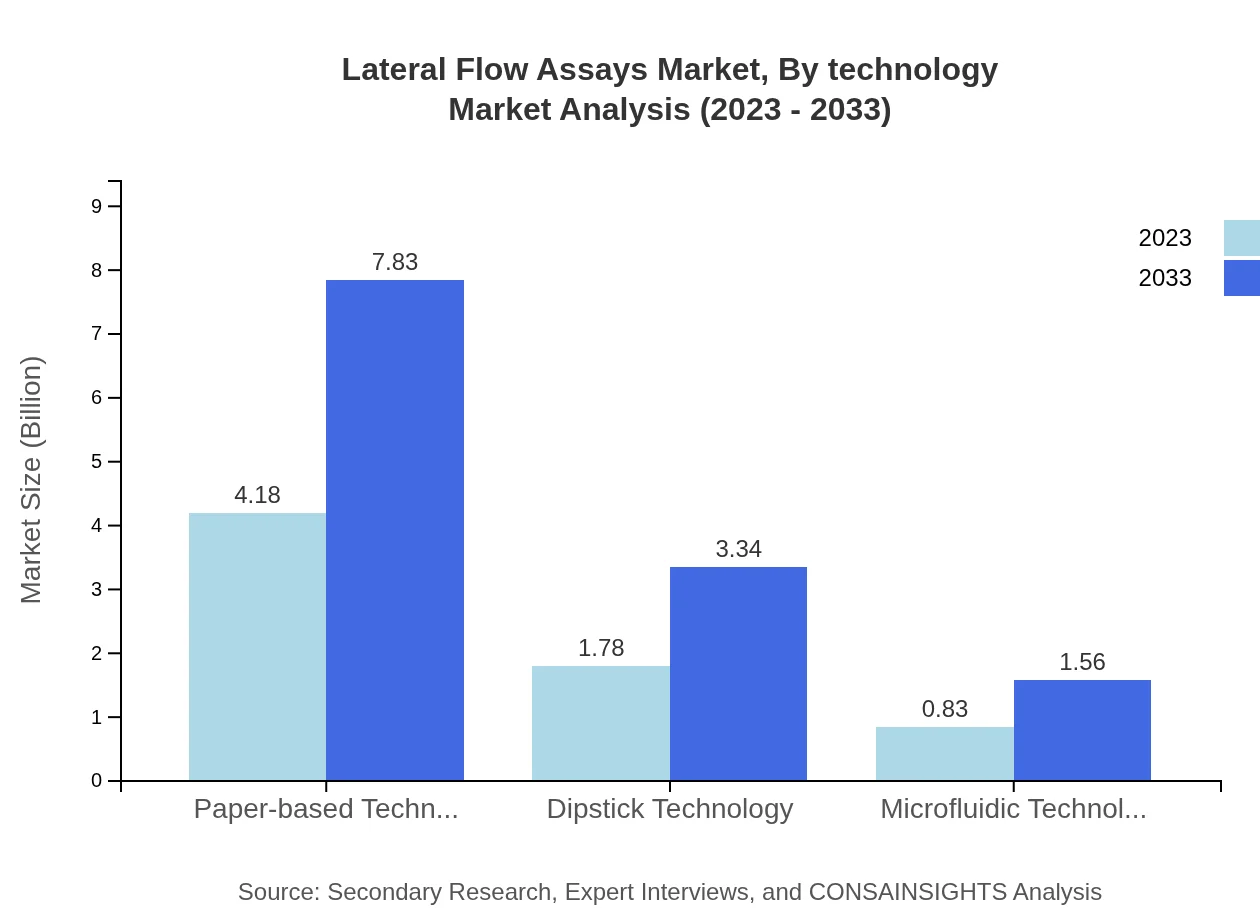
Lateral Flow Assays Market Analysis By Product Type
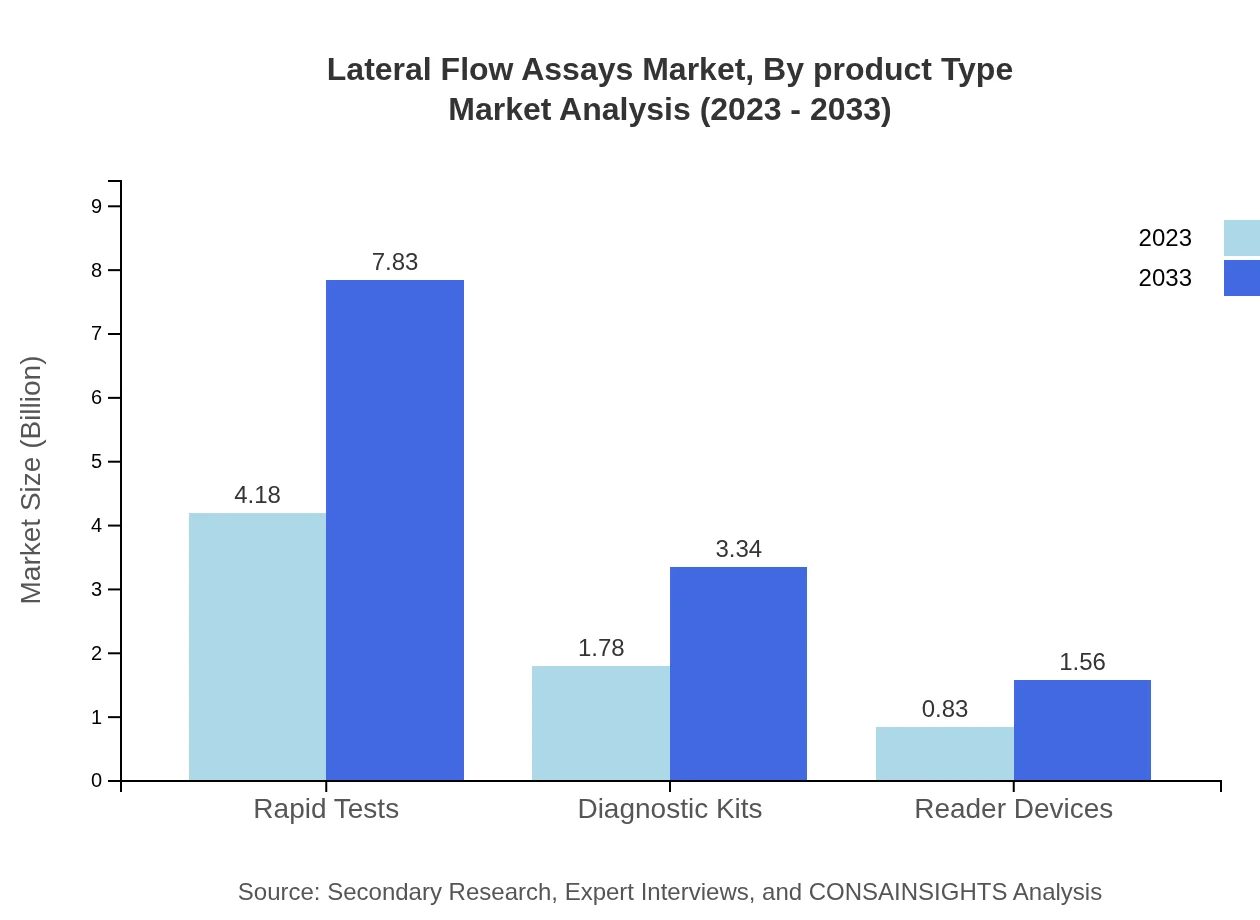
The Lateral Flow Assays market by product type reveals significant trends, where paper-based technology dominates with a size of $4.18 billion in 2023, expanding to $7.83 billion by 2033. Dipstick technology follows, growing from $1.78 billion to $3.34 billion, while microfluidic technology has a modest share, increasing from $0.83 billion to $1.56 billion over the forecast period.
Lateral Flow Assays Market Analysis By Application
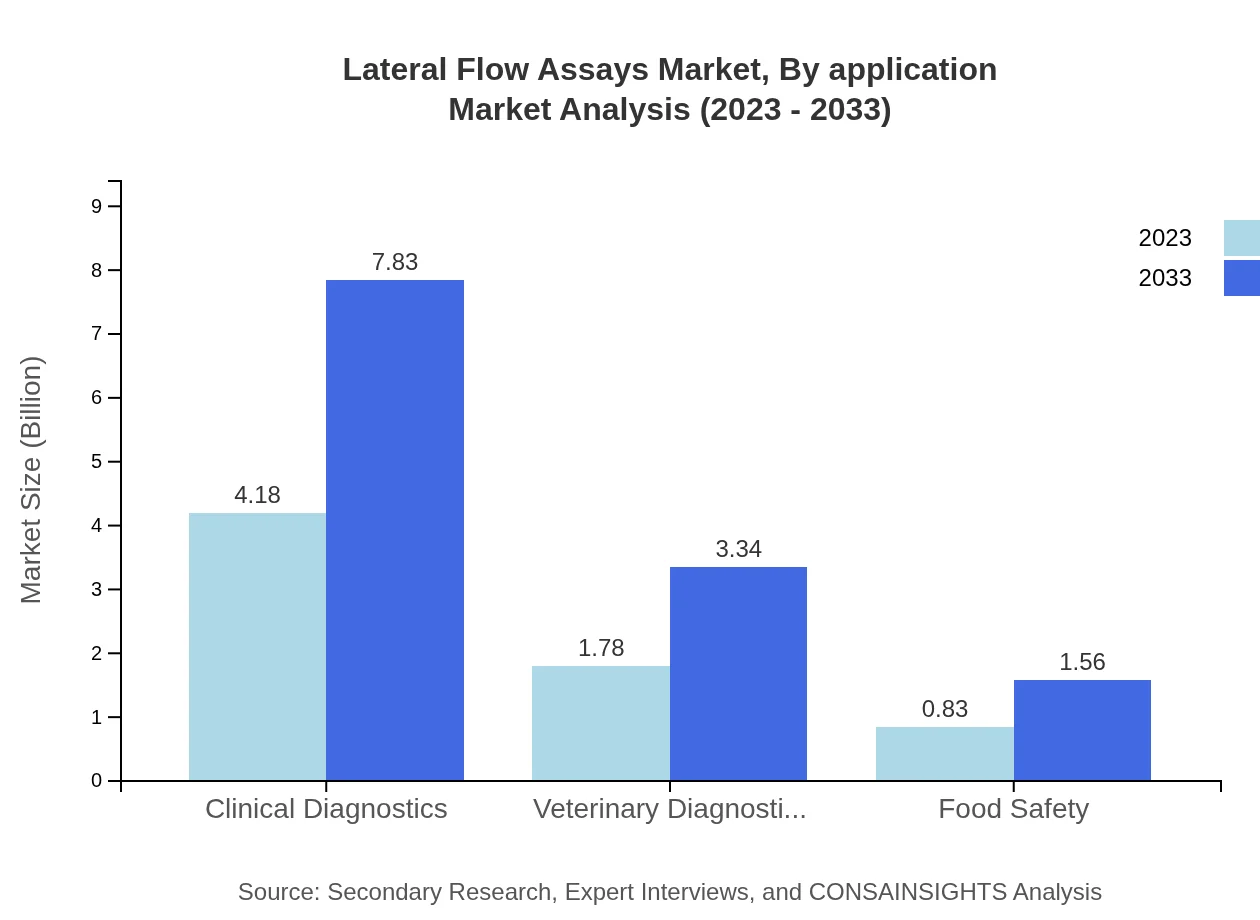
In terms of application, clinical diagnostics constitutes the largest share of the lateral flow assays market, valued at $4.18 billion in 2023; this is projected to rise to $7.83 billion by 2033. Following are veterinary diagnostics, which move from $1.78 billion to $3.34 billion, and food safety applications which grow from $0.83 billion to $1.56 billion during the same period.
Lateral Flow Assays Market Analysis By End User
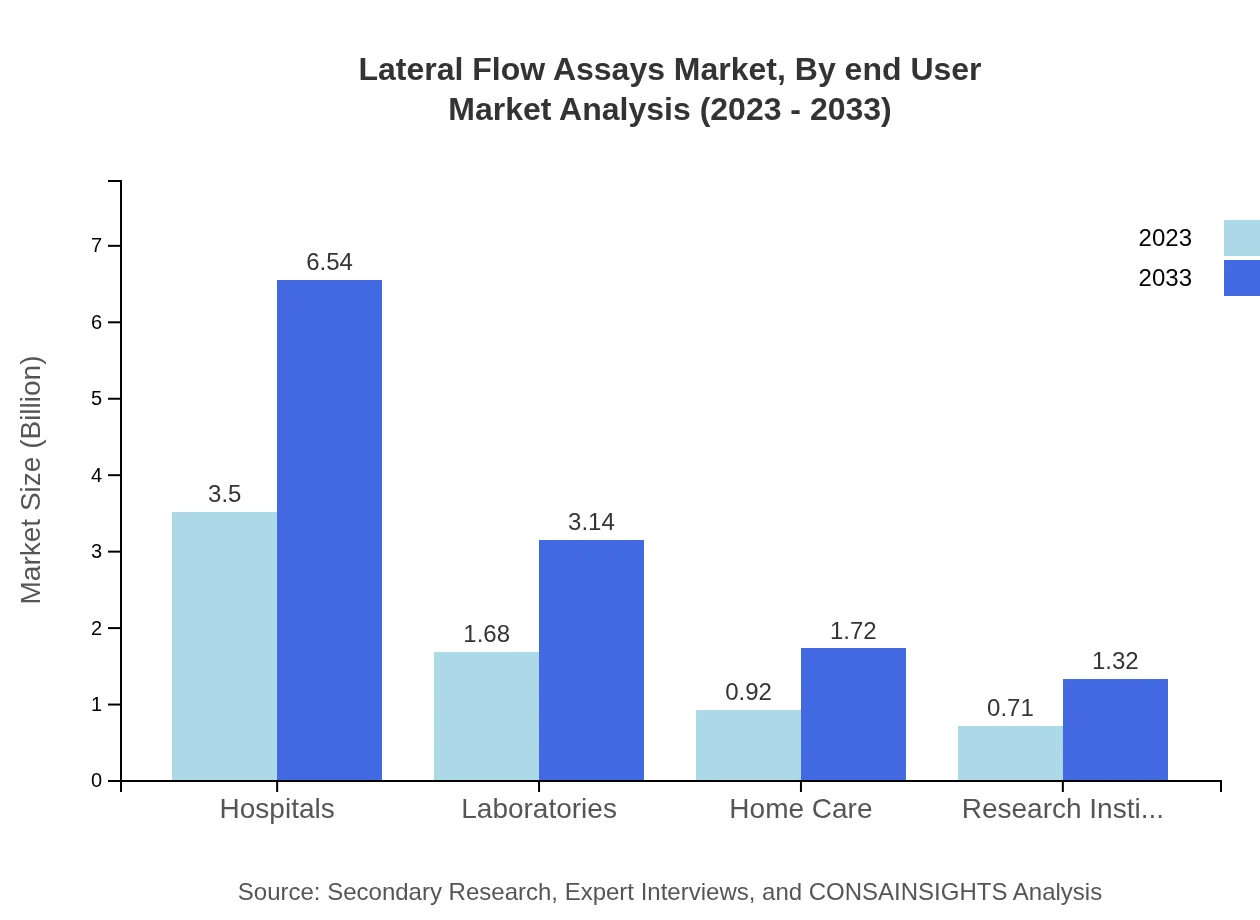
Analysis by end-user indicates that hospitals will continue to hold the largest market size in lateral flow assays, with a value of $3.50 billion in 2023 and growing to $6.54 billion by 2033. Laboratories and home care settings also represent important segments, projected to reach $1.68 billion and $0.92 billion respectively by 2033.
Lateral Flow Assays Market Analysis By Region
Regional analysis highlights North America leading the market with a share of 61.52% in 2023, maintaining this position into 2033, while Europe holds 26.25% and Asia Pacific accounts for 12.23% of the global market, reflecting the prominent role of established healthcare systems in these regions.
Lateral Flow Assays Market Analysis By Technology
Technology-wise, the lateral flow assays market is primarily driven by rapid tests technology, which accounts for 61.52% of the market share in 2023, expected to sustain this lead through 2033. Other technologies, including dipstick and microfluidic, have considerable shares contributing to the market dynamics.
Lateral Flow Assays Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Lateral Flow Assays Industry
Abbott Laboratories:
A leading company in diagnostic products, known for its innovative lateral flow assays that are integral in clinical diagnostics and point-of-care testing.Roche Diagnostics:
Specializes in providing advanced laboratory and diagnostic solutions, significant player in the LFA market with its well-established product lines.Thermo Fisher Scientific:
A prominent leader in the life sciences field, offers a wide range of lateral flow assay products for various applications including clinical, veterinary, and environmental diagnostics.Abingdon Health:
Focused on developing rapid lateral flow tests with high sensitivity and specificity for medical and non-medical applications.We're grateful to work with incredible clients.









FAQs
What is the market size of lateral Flow Assays?
The lateral-flow assays market is valued at approximately $6.8 billion in 2023, with a projected compound annual growth rate (CAGR) of 6.3% up to 2033, reflecting a robust growth trajectory in this industry.
What are the key market players or companies in this lateral Flow Assays industry?
Key players in the lateral-flow assays market include established diagnostics companies, innovative biotechnology firms, and pharmaceutical giants specializing in rapid testing solutions across various applications, although specific names can't be disclosed in this format.
What are the primary factors driving the growth in the lateral Flow Assays industry?
Factors such as the increasing demand for rapid diagnostic solutions, advancements in technology, the rise of home testing, and the growth in infectious diseases are significantly driving the growth of the lateral-flow assays industry.
Which region is the fastest Growing in lateral Flow Assays?
The Asia Pacific region is noted as the fastest-growing market for lateral-flow assays, expected to grow from $1.13 billion in 2023 to approximately $2.12 billion by 2033.
Does ConsaInsights provide customized market report data for the lateral Flow Assays industry?
Yes, ConsaInsights offers customized market report data tailored to clients' specific needs, ensuring detailed insights and analyses that cater to various aspects of the lateral-flow assays industry.
What deliverables can I expect from this lateral Flow Assays market research project?
Deliverables include comprehensive market analysis reports, segmentation data, growth forecasts, and competitive landscape evaluations tailored to the lateral-flow assays market, ensuring actionable insights for strategic planning.
What are the market trends of lateral Flow Assays?
Current market trends include increasing adoption of paper-based technologies, rising demand for home care testing, and shifts toward microfluidic technology, all indicating a progressive evolution in the lateral-flow assays landscape.