Left Atrial Appendage Laa Closure Devices Market Report
Published Date: 31 January 2026 | Report Code: left-atrial-appendage-laa-closure-devices
Left Atrial Appendage Laa Closure Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Left Atrial Appendage Laa Closure Devices market, with insights into market size, industry trends, regional analysis, and forecasts from 2023 to 2033, catering to healthcare professionals and investors.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
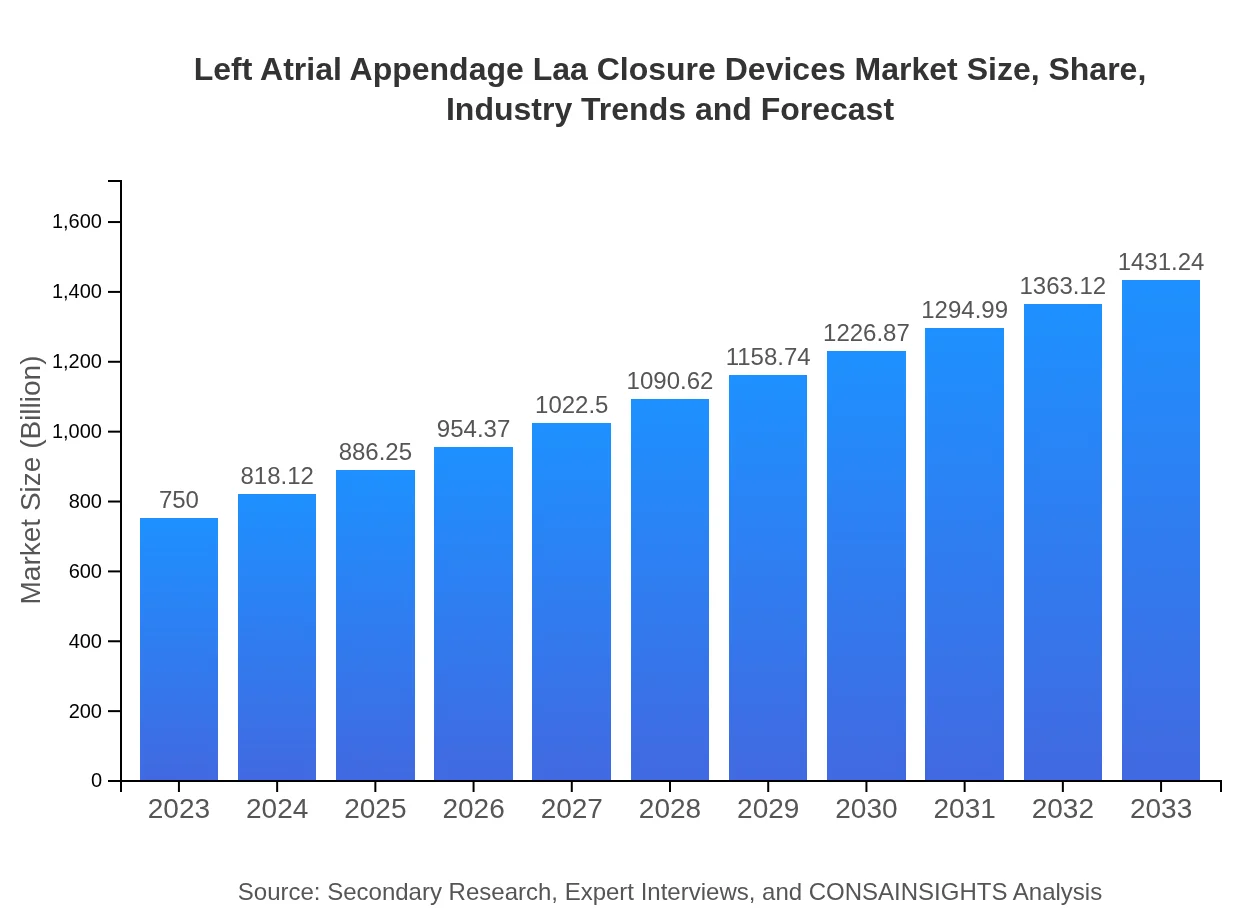
| 2023 Market Size | $750.00 Million |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $1431.24 Million |
| Top Companies | Boston Scientific Corporation, Abbott Laboratories, Medtronic PLC |
| Last Modified Date | 31 January 2026 |
Left Atrial Appendage Laa Closure Devices Market Overview
Customize Left Atrial Appendage Laa Closure Devices Market Report market research report
- ✔ Get in-depth analysis of Left Atrial Appendage Laa Closure Devices market size, growth, and forecasts.
- ✔ Understand Left Atrial Appendage Laa Closure Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Left Atrial Appendage Laa Closure Devices
What is the Market Size & CAGR of Left Atrial Appendage Laa Closure Devices market in 2023?
Left Atrial Appendage Laa Closure Devices Industry Analysis
Left Atrial Appendage Laa Closure Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Left Atrial Appendage Laa Closure Devices Market Analysis Report by Region
Europe Left Atrial Appendage Laa Closure Devices Market Report:
In Europe, the market is anticipated to increase from USD 190.57 million in 2023 to USD 363.68 million by 2033. The region's growth is propelled by increasing prevalence of cardiovascular diseases and adoption of innovative closure devices.Asia Pacific Left Atrial Appendage Laa Closure Devices Market Report:
In the Asia Pacific region, the market for Left Atrial Appendage Closure Devices is projected to grow from USD 144.67 million in 2023 to USD 276.09 million by 2033. Factors include the increasing incidence of atrial fibrillation, improved healthcare infrastructure, and rising awareness of cardiac health.North America Left Atrial Appendage Laa Closure Devices Market Report:
North America holds the largest share in the LAA Closure Devices market, projected to grow from USD 267.38 million in 2023 to USD 510.24 million by 2033. This growth is driven by high healthcare expenditures, presence of key market players, and advanced healthcare facilities.South America Left Atrial Appendage Laa Closure Devices Market Report:
The South American market is expected to expand from USD 63.23 million in 2023 to USD 120.65 million by 2033, primarily due to growing populations and investments in medical facilities that promote advanced treatment options.Middle East & Africa Left Atrial Appendage Laa Closure Devices Market Report:
The Middle East and Africa market is estimated to grow from USD 84.15 million in 2023 to USD 160.59 million by 2033, aided by expanding healthcare initiatives and importation of advanced medical technologies.Tell us your focus area and get a customized research report.
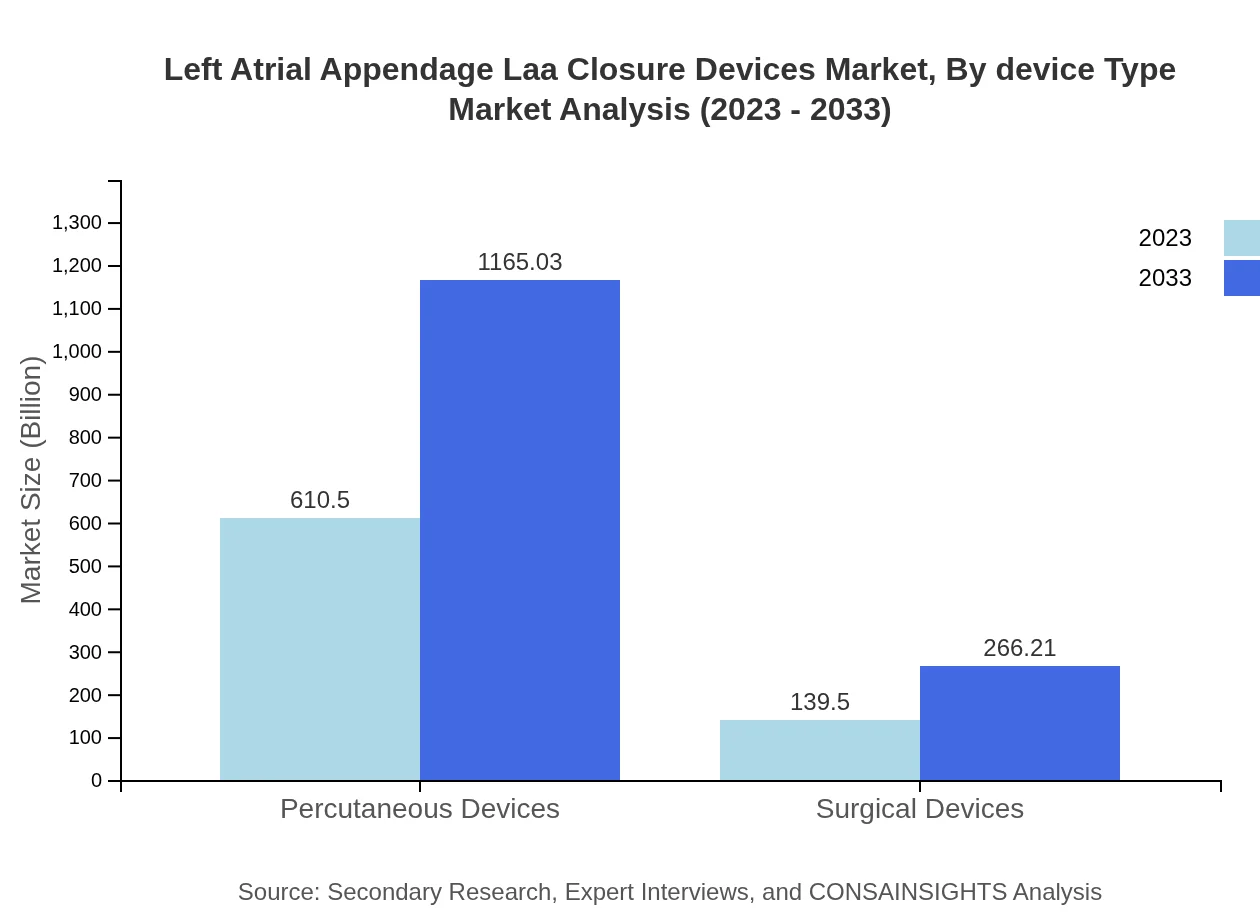
Left Atrial Appendage Laa Closure Devices Market Analysis By Device Type
In 2023, the transcatheter technique segment dominates the market, valued at USD 610.50 million, and is anticipated to reach USD 1165.03 million by 2033. The minimally invasive nature of transcatheter devices contributes to their increased preference. Conversely, surgical techniques account for USD 139.50 million and project similar growth, marking a gradual shift towards non-invasive procedures.
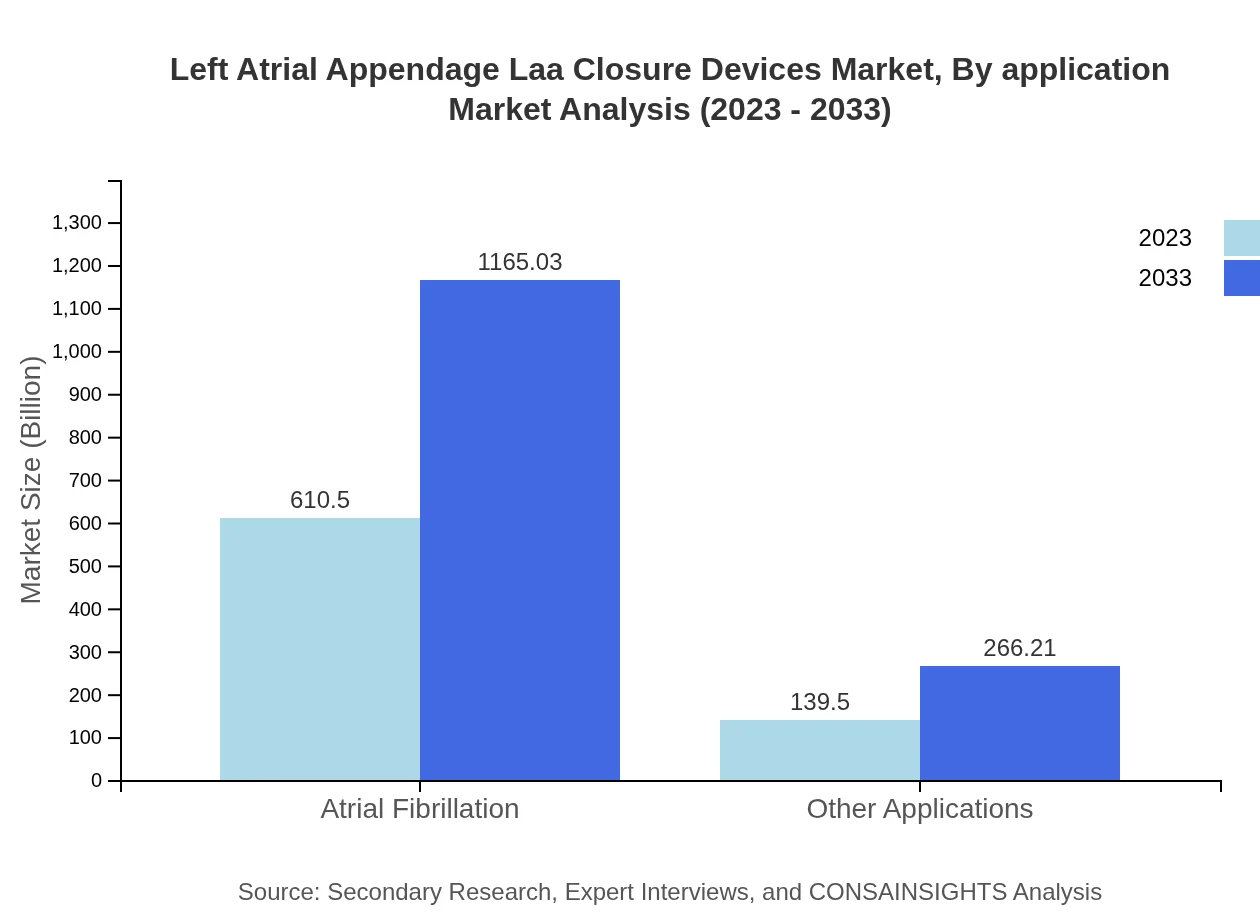
Left Atrial Appendage Laa Closure Devices Market Analysis By Application
The application of LAA closure devices for atrial fibrillation constitutes the majority share, with the segment valued at USD 610.50 million in 2023, projected to grow to USD 1165.03 million by 2033. Other applications hold a smaller market share, indicating a focused healthcare approach towards AF-related interventions.
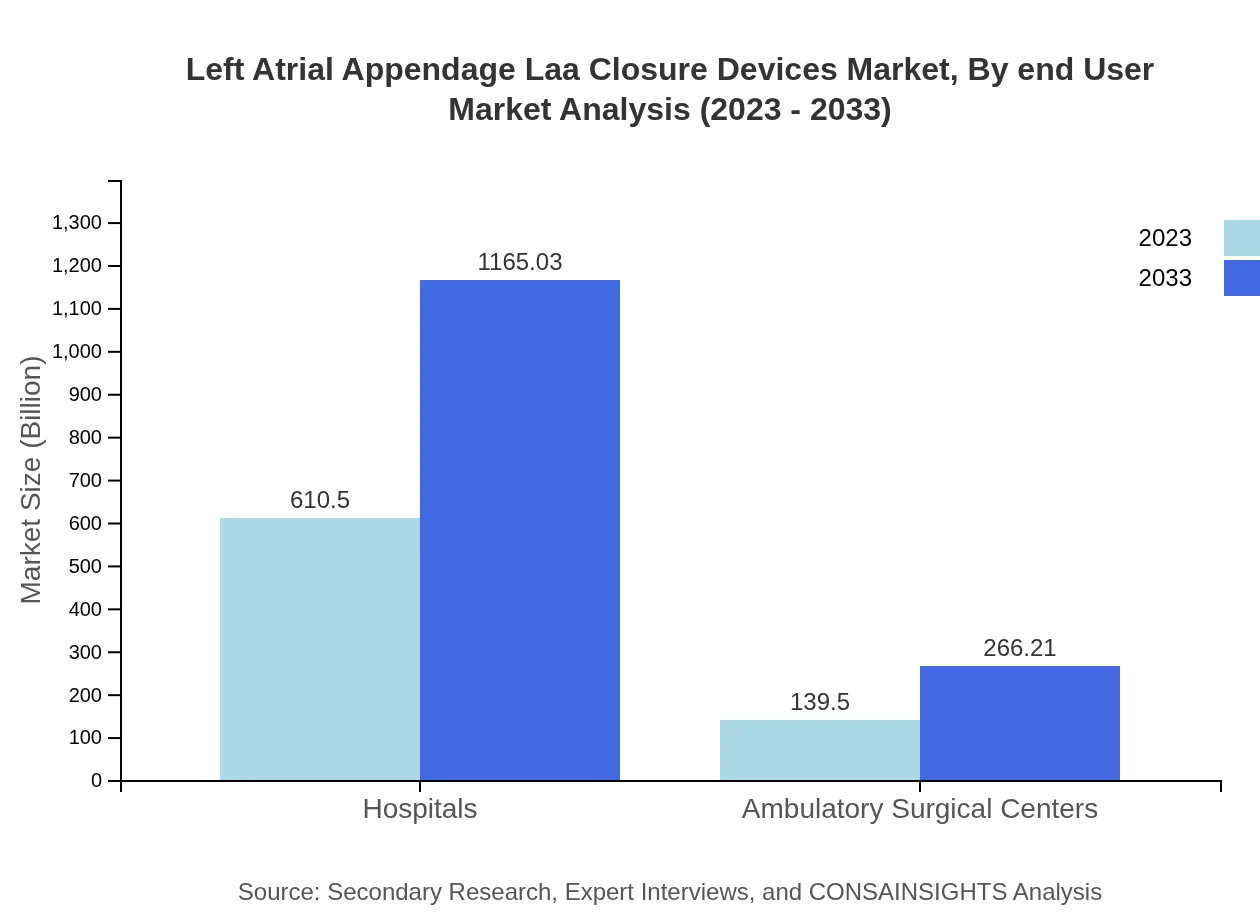
Left Atrial Appendage Laa Closure Devices Market Analysis By End User
Hospitals continue to be the dominant end-user of LAA closure devices, valued at USD 610.50 million in 2023, with projections suggesting growth to USD 1165.03 million by 2033. Ambulatory surgical centers, though smaller in market size at USD 139.50 million, also show significant growth potential.
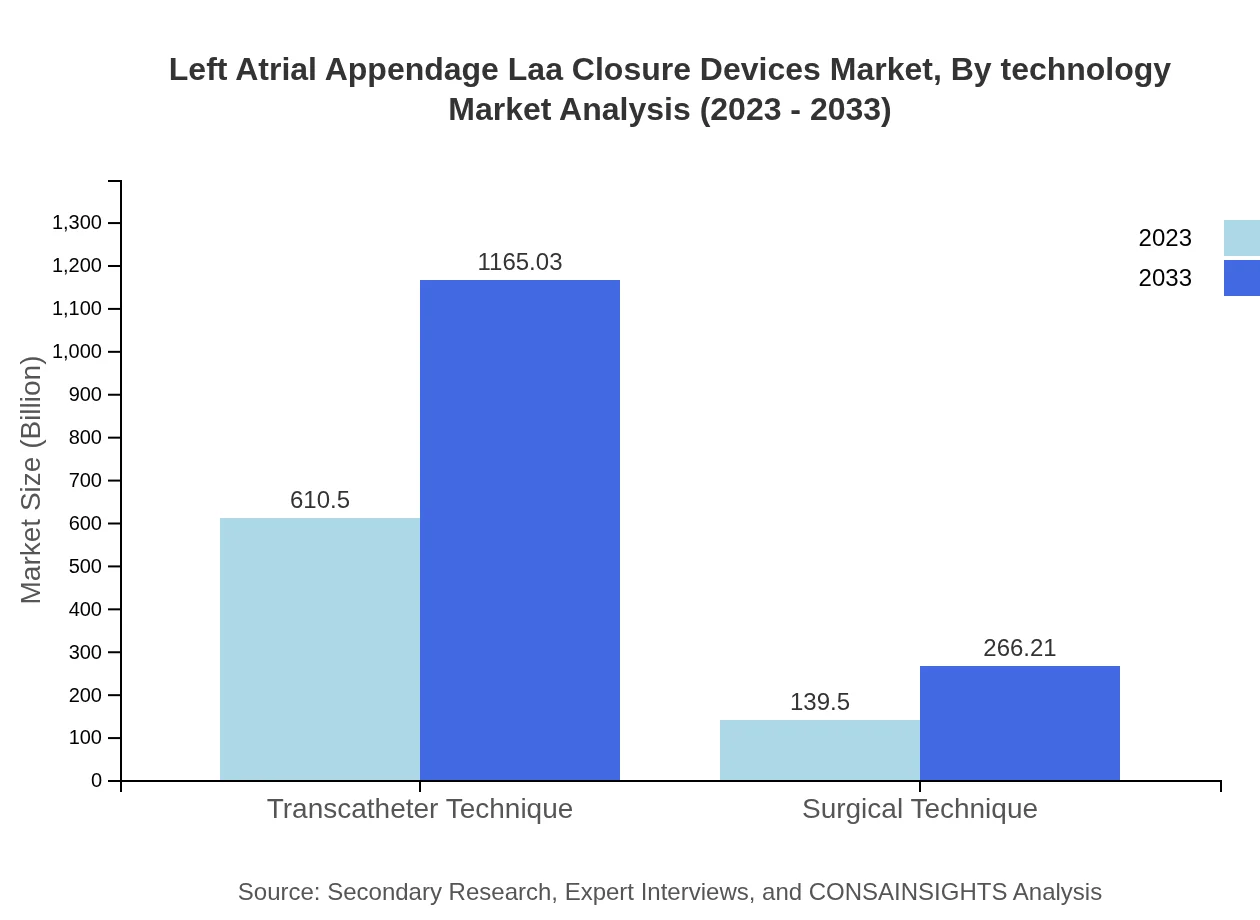
Left Atrial Appendage Laa Closure Devices Market Analysis By Technology
The market is increasingly inclined toward percutaneous devices, which stand at USD 610.50 million in 2023, with an expected rise to USD 1165.03 million by 2033, reflecting growing trends in less invasive surgical preferences. Surgical devices hold a market size value of USD 139.50 million, indicating innovation in that sector as well.
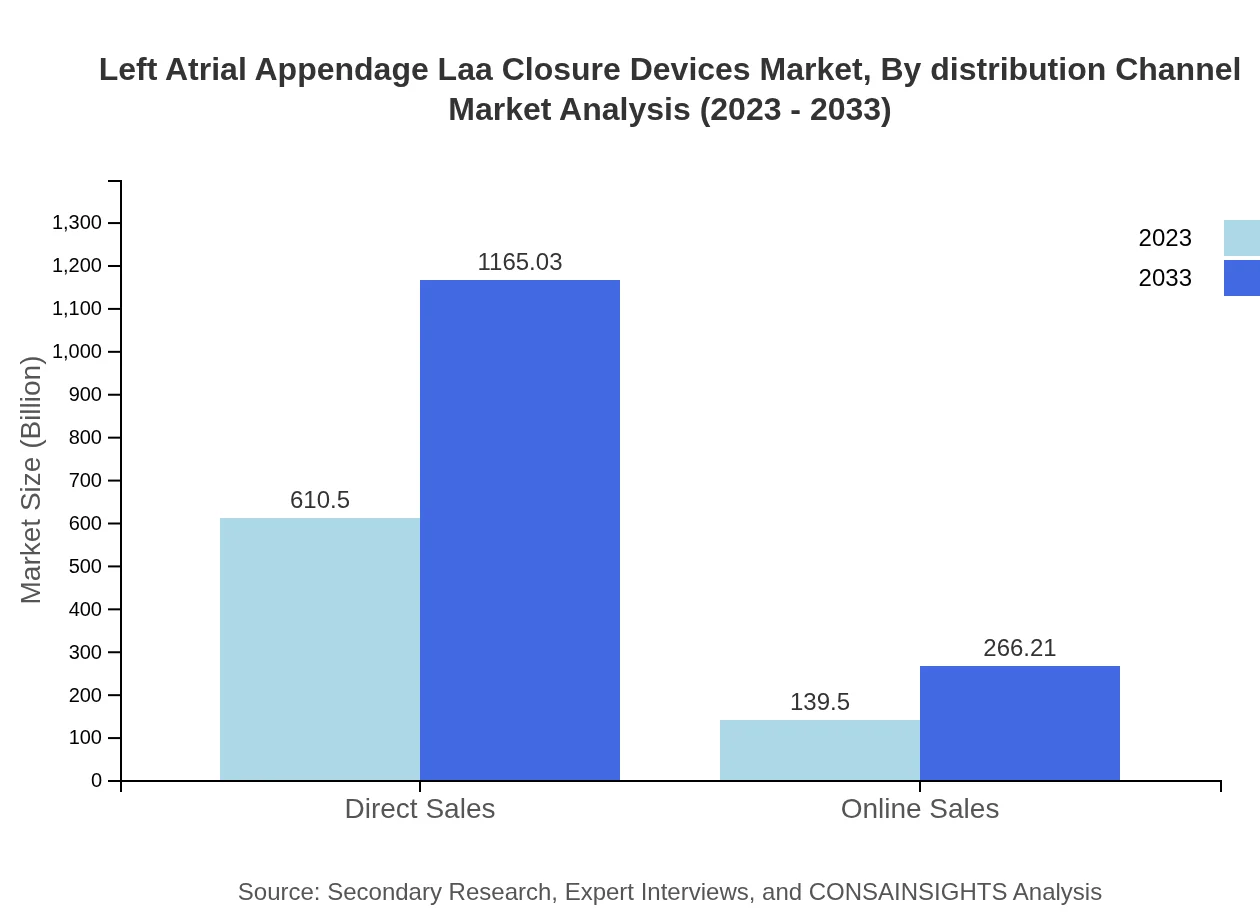
Left Atrial Appendage Laa Closure Devices Market Analysis By Distribution Channel
Direct sales channels lead with a robust market value of USD 610.50 million and escalate to USD 1165.03 million by 2033, supported by strong manufacturer-consumer relationships. Online sales, while less impactful at USD 139.50 million, signify the growing trend toward e-commerce in the medical field.
Left Atrial Appendage Laa Closure Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Left Atrial Appendage Laa Closure Devices Industry
Boston Scientific Corporation:
A leading global medical device manufacturer recognized for its innovative LAA closure devices, improving patient outcomes and operational efficacy in cardiac procedures.Abbott Laboratories:
Known for developing the Amplatzer device, Abbott continues to pioneer in the LAA closure market through research and partnerships that expand device capabilities.Medtronic PLC:
Medtronic is a market leader specializing in advanced cardiovascular solutions, contributing significantly through its innovative closure devices and strong global distribution networks.We're grateful to work with incredible clients.









FAQs
What is the market size of left Atrial Appendage Laa Closure Devices?
The left atrial appendage (LAA) closure devices market is estimated to be around $750 million in 2023, with a projected CAGR of 6.5%. This growth signifies the increasing demand for innovative cardiology solutions.
What are the key market players or companies in the left Atrial Appendage Laa Closure Devices industry?
Key players in the LAA closure devices market include major medical technology companies focusing on cardiac devices. These companies innovate new technologies to enhance treatment options for patients suffering from atrial fibrillation and related conditions.
What are the primary factors driving the growth in the left atrial appendage closure devices industry?
Growth in the LAA closure devices market is driven by rising atrial fibrillation cases, advancements in minimally invasive surgeries, increased healthcare expenditures, and a growing emphasis on improving patient outcomes and reducing stroke risks associated with AF.
Which region is the fastest Growing in the left atrial appendage closure devices?
The Asia Pacific region is identified as the fastest-growing market for left atrial appendage closure devices, with market growth projections indicating a rise from $144.67 million in 2023 to $276.09 million by 2033, owing to improved healthcare access.
Does ConsaInsights provide customized market report data for the left atrial appendage closure devices industry?
Yes, Consainsights offers customized market report data tailored to the specific needs of clients within the left atrial appendage closure devices industry, ensuring relevant insights and actionable data for decision-making.
What deliverables can I expect from this left atrial appendage closure devices market research project?
Expect comprehensive deliverables such as market size data, growth forecasts, competitive analysis, regional insights, trends, and segmentation information, all designed to assist stakeholders in strategic planning and investment decisions.
What are the market trends of left atrial appendage closure devices?
Current trends in the left atrial appendage closure devices market include the shift towards minimally invasive procedures, technological advancements in device design and functionality, and increased patient awareness and acceptance of these innovative treatments.