Mirna Sequencing And Assay Market Report
Published Date: 31 January 2026 | Report Code: mirna-sequencing-and-assay
Mirna Sequencing And Assay Market Size, Share, Industry Trends and Forecast to 2033
This report presents a comprehensive analysis of the Mirna Sequencing and Assay market, providing valuable insights into market size, trends, and forecasts from 2023 to 2033, along with industry-specific challenges and opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
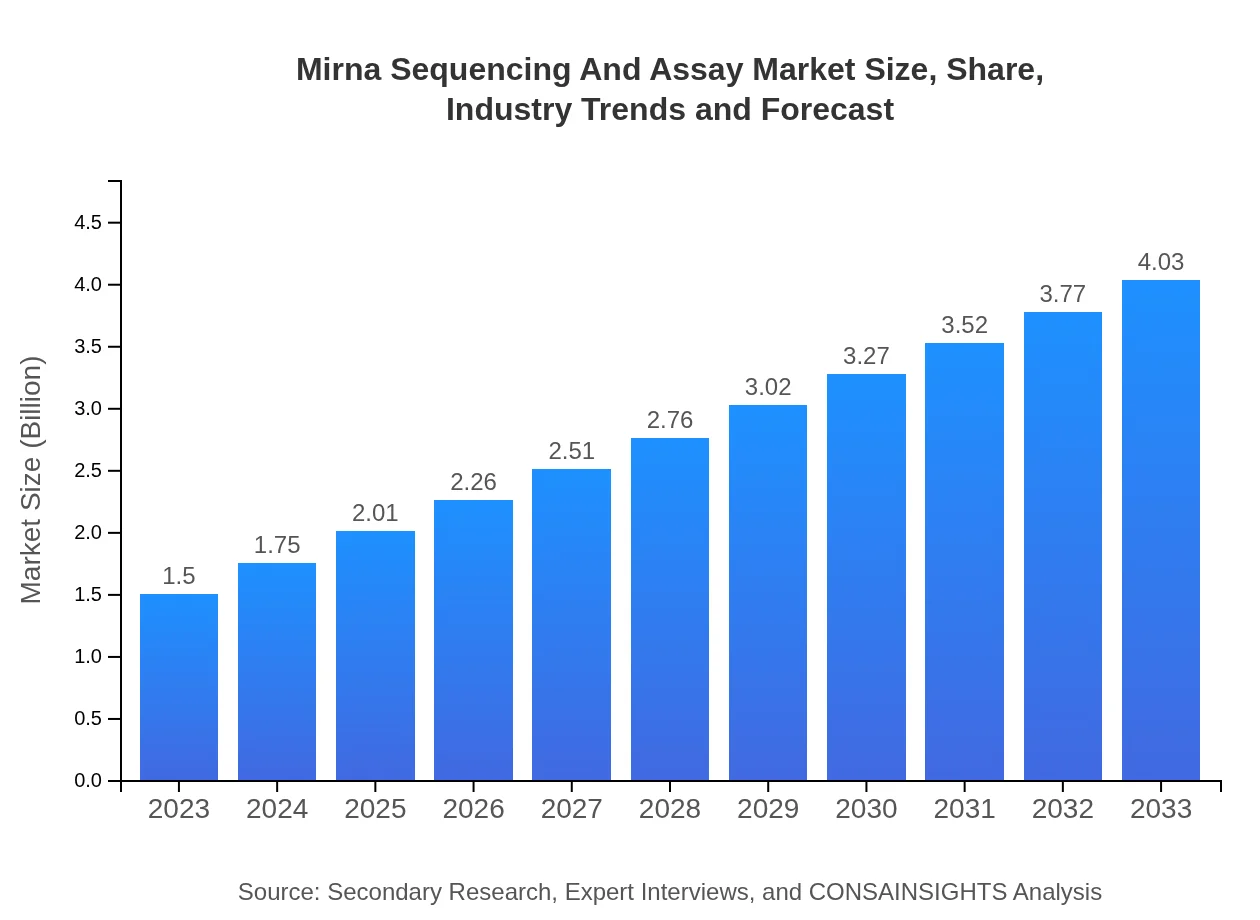
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 10% |
| 2033 Market Size | $4.03 Billion |
| Top Companies | Illumina, Inc., Thermo Fisher Scientific Inc., Qiagen N.V., Agilent Technologies, Inc. |
| Last Modified Date | 31 January 2026 |
Mirna Sequencing And Assay Market Overview
Customize Mirna Sequencing And Assay Market Report market research report
- ✔ Get in-depth analysis of Mirna Sequencing And Assay market size, growth, and forecasts.
- ✔ Understand Mirna Sequencing And Assay's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Mirna Sequencing And Assay
What is the Market Size & CAGR of Mirna Sequencing And Assay market in 2033?
Mirna Sequencing And Assay Industry Analysis
Mirna Sequencing And Assay Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Mirna Sequencing And Assay Market Analysis Report by Region
Europe Mirna Sequencing And Assay Market Report:
The European market is expected to witness growth from $0.43 billion in 2023 to $1.16 billion by 2033. The increase is primarily driven by strong governmental support for genomic research and collaborations between biotechnology firms and academic institutions that foster miRNA research applications.Asia Pacific Mirna Sequencing And Assay Market Report:
In the Asia Pacific region, the Mirna Sequencing and Assay market is expected to grow from $0.28 billion in 2023 to $0.74 billion in 2033, driven by increasing research and development activities and collaboration between research organizations and healthcare entities. The expanding healthcare infrastructure and advancements in sequencing technologies further support this growth.North America Mirna Sequencing And Assay Market Report:
North America holds a substantial market share, anticipated to grow from $0.58 billion in 2023 to $1.55 billion in 2033. The expansion in this region is primarily due to advanced healthcare systems, increased research funding, and a higher prevalence of chronic diseases necessitating advanced diagnostics and therapeutic interventions.South America Mirna Sequencing And Assay Market Report:
The South American market is projected to expand from $0.14 billion in 2023 to $0.37 billion by 2033. The growth is facilitated through rising investments in biotechnology research, health facilities, and awareness about personalized medicine, which is propelling the miRNA applications in various clinical scenarios.Middle East & Africa Mirna Sequencing And Assay Market Report:
The Middle East and Africa region shows market growth from $0.07 billion in 2023 to $0.20 billion by 2033. Although this region is in the nascent stage of miRNA research, rising awareness regarding advanced diagnostic techniques and exploration of genetic research opportunities are driving modest market growth.Tell us your focus area and get a customized research report.
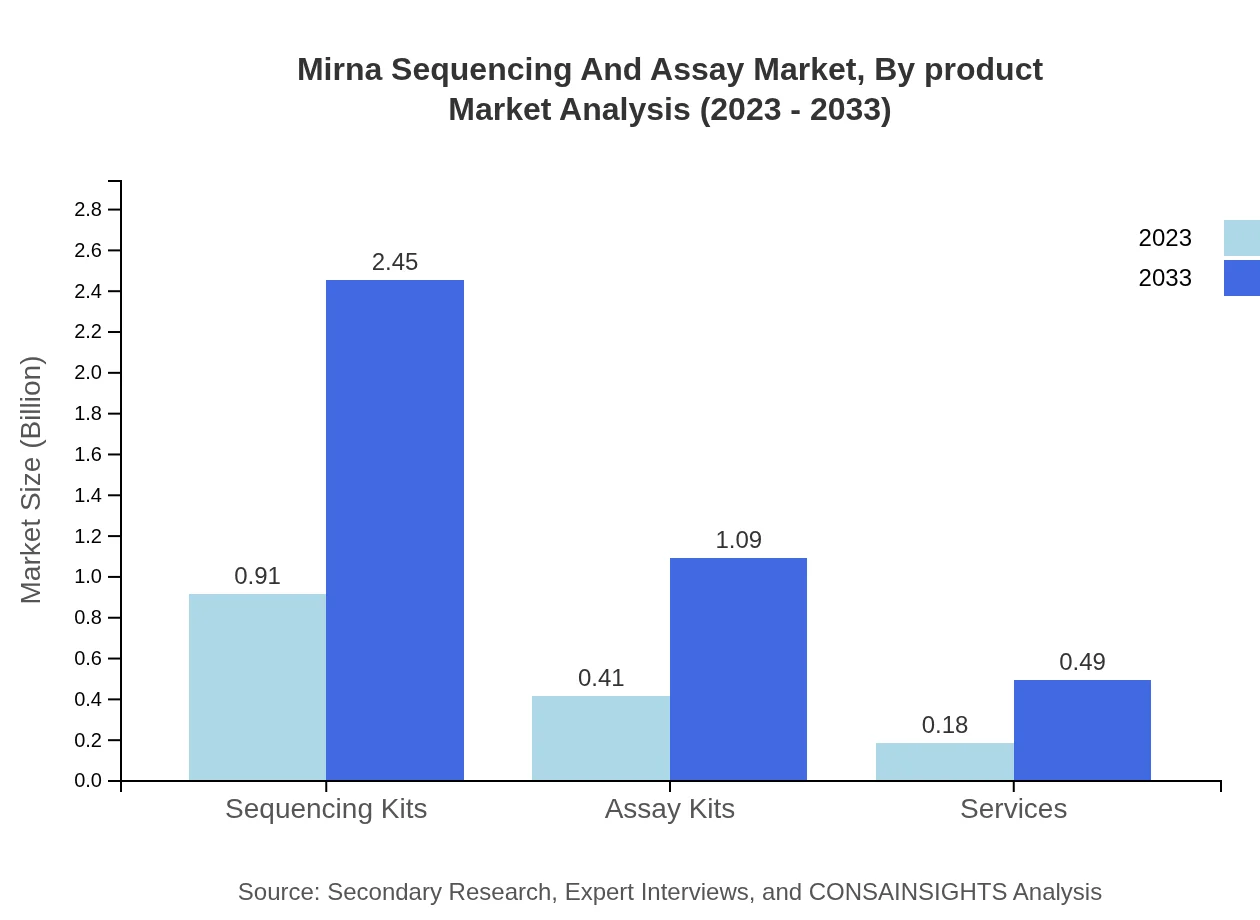
Mirna Sequencing And Assay Market Analysis By Product
In 2023, the Sequencing Kits segment accounted for $0.91 billion, which is 60.87% of the market share, projected to grow to $2.45 billion by 2033. Assay Kits hold $0.41 billion, marking 27.03% share, expected to reach $1.09 billion. Services constitute $0.18 billion contributing to a 12.1% share, anticipating growth to $0.49 billion.
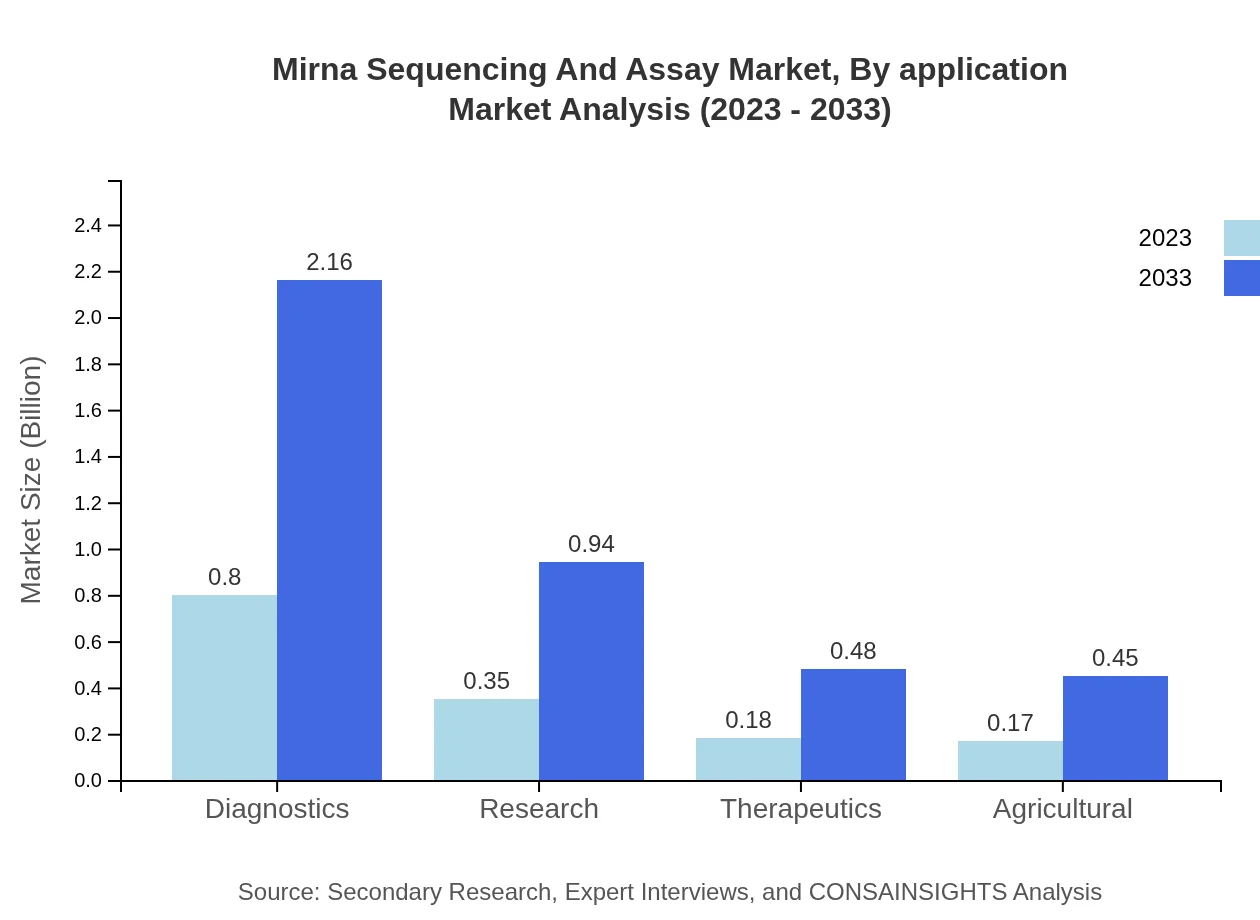
Mirna Sequencing And Assay Market Analysis By Application
Diagnostics, with a market share of 53.56% worth $0.80 billion, is expected to reach $2.16 billion by 2033, while Research holds a share of 23.45% at $0.35 billion, projected to grow to $0.94 billion. The Therapeutics application segment, currently at 11.86% ($0.18 billion), will grow to $0.48 billion by 2033.
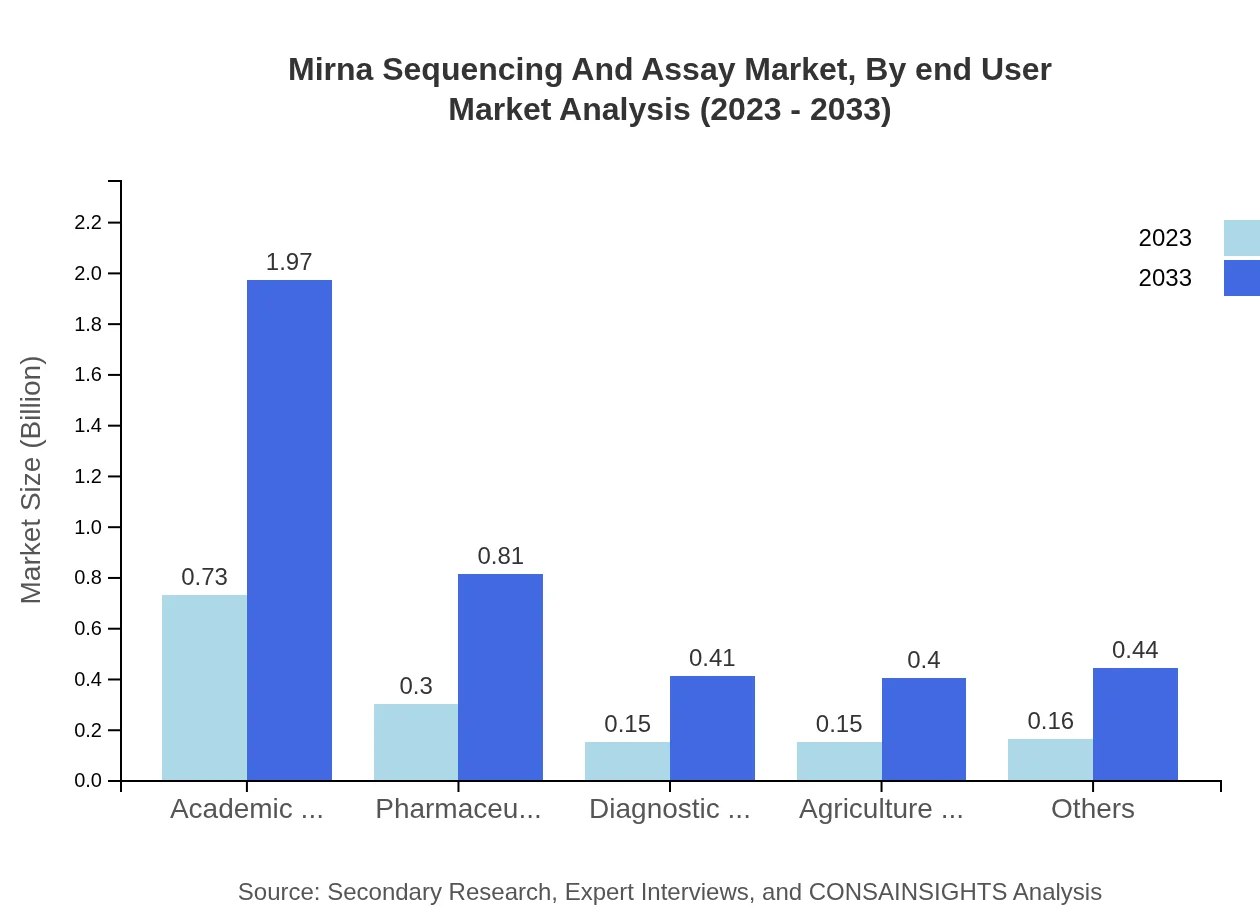
Mirna Sequencing And Assay Market Analysis By End User
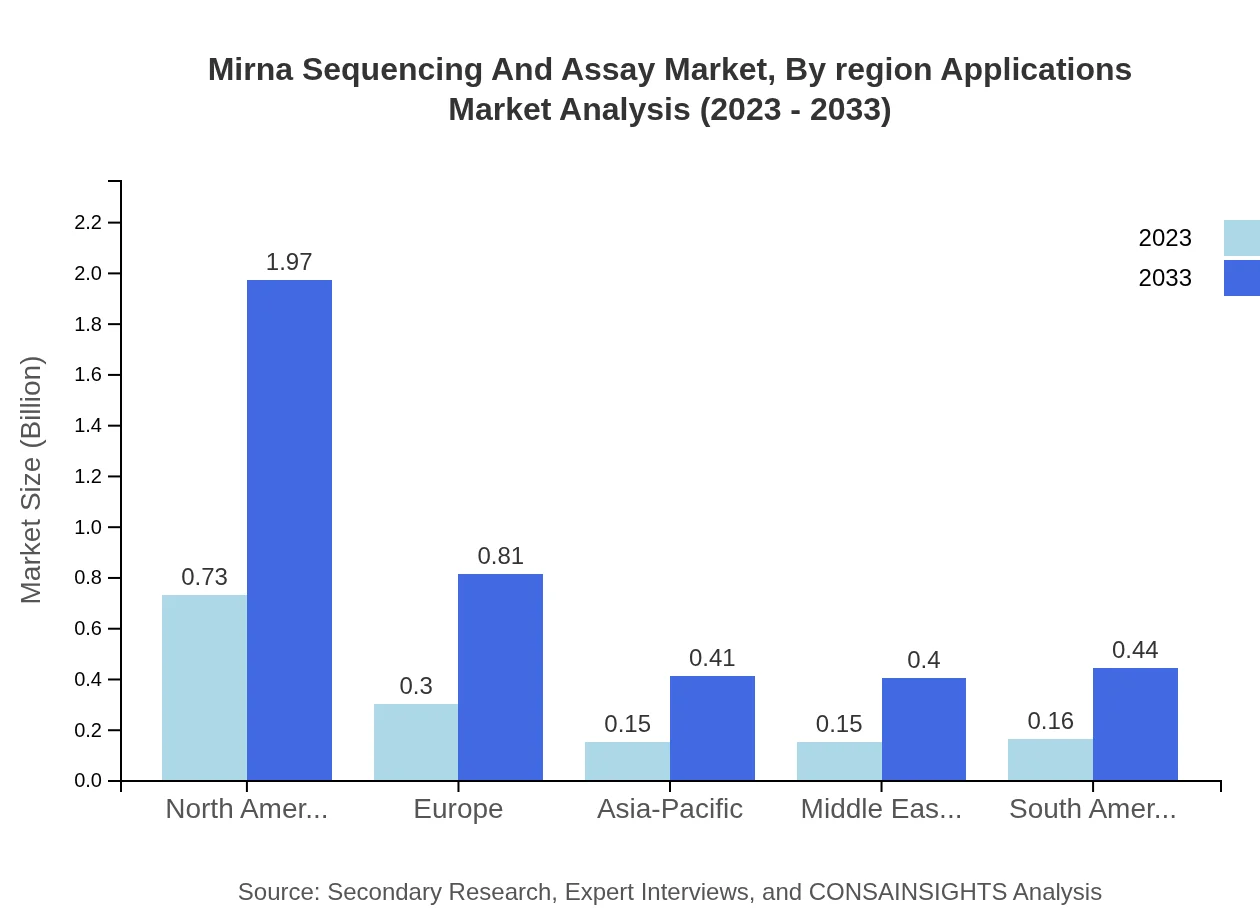
The Academic and Research Institutes segment is valued at $0.73 billion, comprising 48.9%, projected to reach $1.97 billion by 2033. Pharmaceutical and Biotechnology Companies currently at 20.03% ($0.30 billion) will grow to $0.81 billion. Diagnostic Laboratories hold 10.07% ($0.15 billion), and Agriculture and Plant Research Companies occupy a share of 10.04% ($0.15 billion).
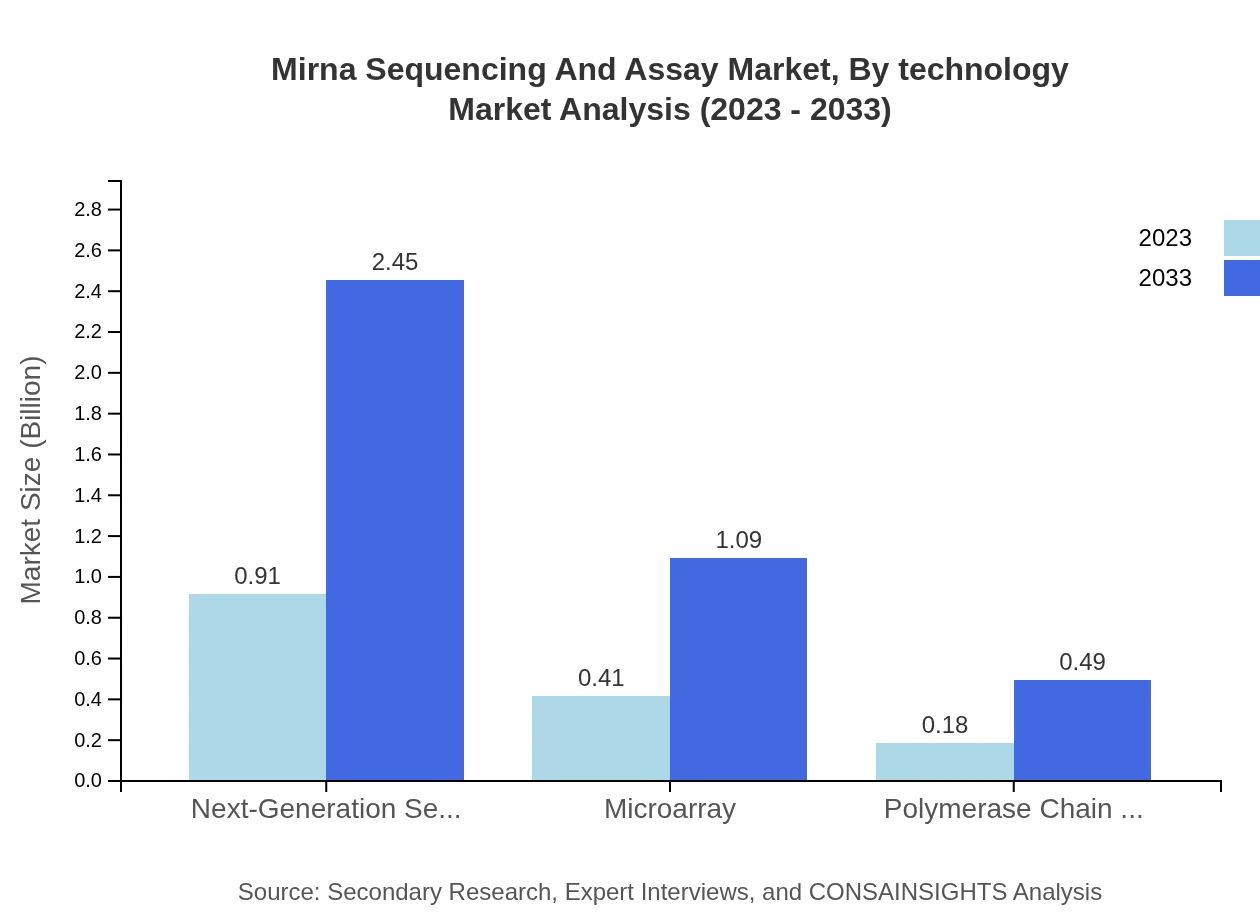
Mirna Sequencing And Assay Market Analysis By Technology
Next-Generation Sequencing (NGS) dominates the sector with a $0.91 billion share, approximately 60.87%, with projections to increase to $2.45 billion by 2033. Microarray technology, holding $0.41 billion (27.03%) is expected to reach $1.09 billion. Polymerase Chain Reaction (PCR), valued at $0.18 billion (12.1%), will expand to $0.49 billion.
Mirna Sequencing And Assay Market Analysis By Region Applications
Excluding specific applications, the regional segmentation indicates that North America leads the market with significant share and is expected to contribute the highest in market growth, while Europe follows with prominent growth initiatives. Asia-Pacific presents opportunities as investments in biotechnology research are growing, often overshadowed by North America's established market.
Mirna Sequencing And Assay Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Mirna Sequencing And Assay Industry
Illumina, Inc.:
Illumina, Inc. is a frontrunner in sequencing technology and genomics, providing advanced platforms and reagents for miRNA analysis coupled with bioinformatics solutions, thereby enhancing research and application development.Thermo Fisher Scientific Inc.:
Thermo Fisher Scientific is a renowned biotechnology company offering a range of advanced miRNA assays and sequencing kits, playing a crucial role in facilitating genomic research and diagnostics globally.Qiagen N.V.:
Qiagen N.V. specializes in molecular diagnostics and is known for its cutting-edge solutions for miRNA extraction and analysis, significantly impacting clinical diagnosis and therapeutic applications.Agilent Technologies, Inc.:
Agilent Technologies provides a portfolio of miRNA analysis solutions and genomics technologies, with a focus on enhancing research efficiency and accuracy in the detection of miRNAs.We're grateful to work with incredible clients.









FAQs
What is the market size of mirna Sequencing And Assay?
The miRNA sequencing and assay market is projected to reach a size of USD 1.5 billion by 2033, growing at a robust CAGR of 10%. This growth reflects the increasing demand for advanced genetic testing methodologies.
What are the key market players or companies in the mirna Sequencing And Assay industry?
Leading players in the miRNA sequencing and assay market include prominent biotechnology and pharmaceutical companies, specialized genomic research firms, and academic institutions focused on advanced sequencing techniques.
What are the primary factors driving the growth in the mirna Sequencing And Assay industry?
Key drivers for growth include rising demand for personalized medicine, advancements in next-generation sequencing technology, and increasing applications in diagnostics and research, particularly in oncology.
Which region is the fastest Growing in the mirna Sequencing And Assay?
The fastest-growing region in the miRNA sequencing and assay market is North America, projected to grow from USD 0.58 billion in 2023 to USD 1.55 billion by 2033, reflecting a strong healthcare infrastructure and innovation.
Does ConsaInsights provide customized market report data for the mirna Sequencing And Assay industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the miRNA sequencing and assay industry, ensuring relevant insights and actionable intelligence for stakeholders.
What deliverables can I expect from this mirna Sequencing And Assay market research project?
Expect comprehensive deliverables including detailed reports, market analyses, competitive landscapes, and forecasts, along with insights on trends, segments, and key players in the miRNA sequencing and assay market.
What are the market trends of mirna Sequencing And Assay?
Current trends include increasing integration of AI in data analysis, development of cheaper and faster sequencing technologies, and a growing emphasis on multi-omic approaches in research and diagnostics.