Molecular Quality Controls Market Report
Published Date: 31 January 2026 | Report Code: molecular-quality-controls
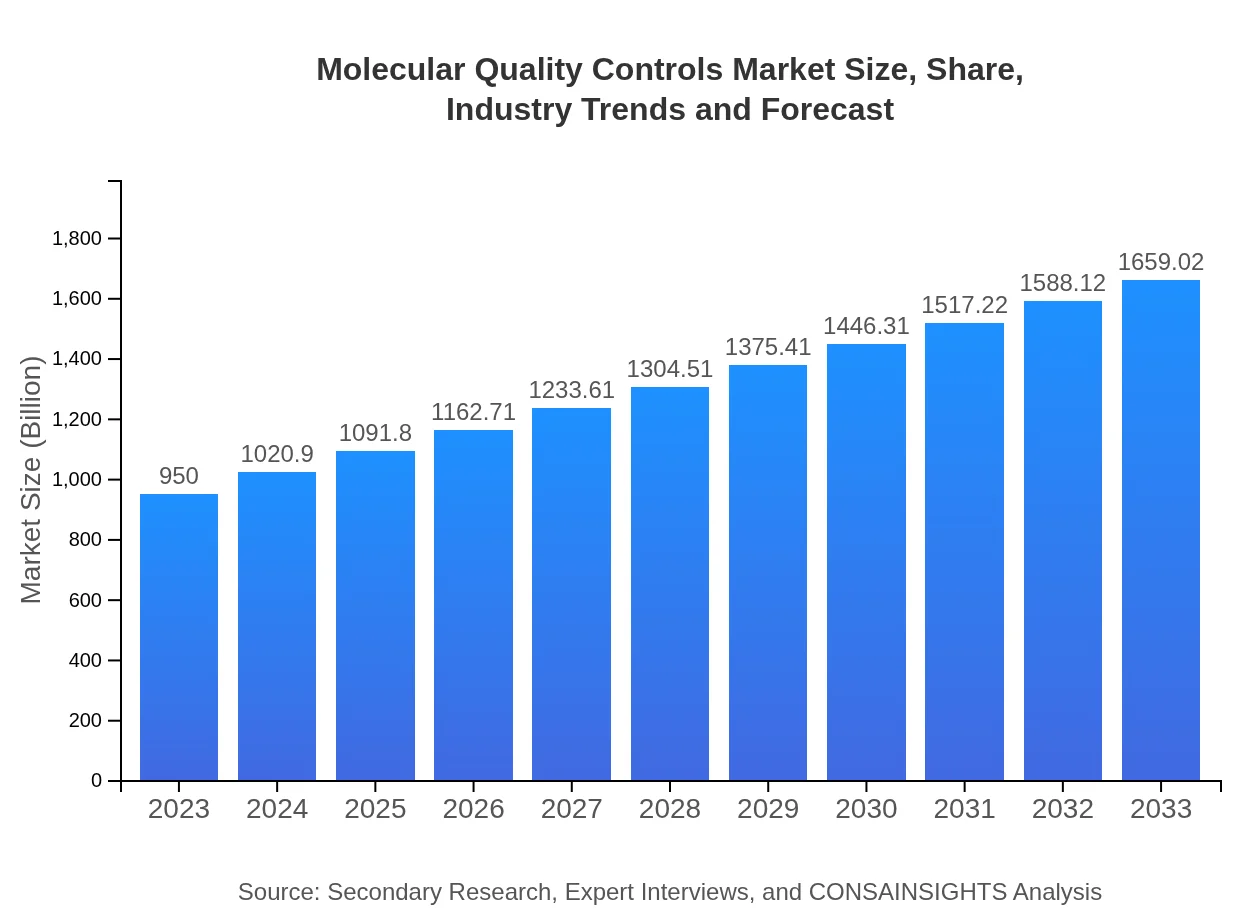
Molecular Quality Controls Market Size, Share, Industry Trends and Forecast to 2033
This report offers an in-depth analysis of the Molecular Quality Controls market, covering key trends, market size, and forecasts from 2023 to 2033, providing insights into market dynamics and influencing factors.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $950.00 Million |
| CAGR (2023-2033) | 5.6% |
| 2033 Market Size | $1659.02 Million |
| Top Companies | Thermo Fisher Scientific, Roche Diagnostics, Bio-Rad Laboratories, Abbott Laboratories, Merck Group |
| Last Modified Date | 31 January 2026 |
Molecular Quality Controls Market Overview
Customize Molecular Quality Controls Market Report market research report
- ✔ Get in-depth analysis of Molecular Quality Controls market size, growth, and forecasts.
- ✔ Understand Molecular Quality Controls's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Molecular Quality Controls
What is the Market Size & CAGR of Molecular Quality Controls market in 2023?
Molecular Quality Controls Industry Analysis
Molecular Quality Controls Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Molecular Quality Controls Market Analysis Report by Region
Europe Molecular Quality Controls Market Report:
Europe's sophisticated healthcare system and high standards of medical testing will continue to drive a robust market for Molecular Quality Controls. The market size in 2023 stands at $301.91 million, expecting growth to $527.24 million by 2033 as a result of increasing demand for personalized medicine and stringent regulatory requirements.Asia Pacific Molecular Quality Controls Market Report:
The Asia Pacific region is characterized by a rapidly growing healthcare infrastructure and increasing investment in biotechnology. In 2023, the market size for Molecular Quality Controls is approximately $155.04 million, projected to reach $270.75 million by 2033, thanks to rising healthcare spending and the increasing prevalence of infectious diseases. This growth is also supported by technology incorporation and burgeoning research activities in molecular diagnostics.North America Molecular Quality Controls Market Report:
North America is anticipated to remain the dominant market, valued at $355.30 million in 2023, projected to climb to $620.47 million by 2033. The growth is driven by advanced research and development activities, increased funding for healthcare innovation, and strong regulatory frameworks that favor quality control practices in laboratory testing.South America Molecular Quality Controls Market Report:
In South America, the Molecular Quality Controls market is at $79.61 million in 2023, with an expected growth to $139.03 million by 2033. The increase is attributed to the rising healthcare initiatives and collaborations between governmental and non-governmental organizations to enhance healthcare quality, thus expanding the use of quality controls in diagnostics.Middle East & Africa Molecular Quality Controls Market Report:
The Middle East and Africa market for Molecular Quality Controls is expected to grow from $58.14 million in 2023 to $101.53 million by 2033, driven by enhanced focus on healthcare access and quality improvements, alongside growing investments in the region’s healthcare infrastructure.Tell us your focus area and get a customized research report.
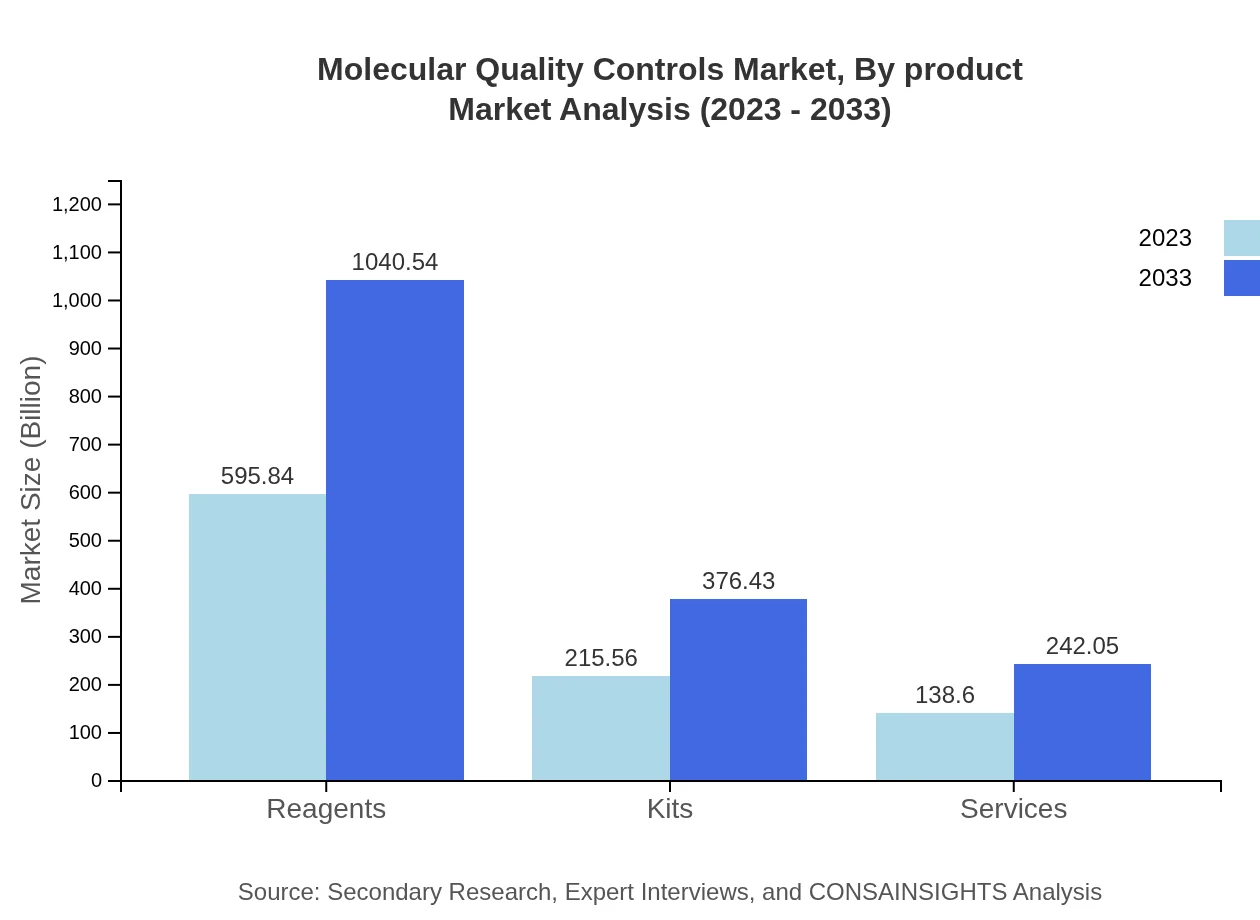
Molecular Quality Controls Market Analysis By Product
By product type, the Molecular Quality Controls market is classified into reagents, kits, and services. In 2023, reagents dominate the market with a valuation of $595.84 million and a projected increase to $1,040.54 million by 2033. Kits follow, projected to rise from $215.56 million in 2023 to $376.43 million in 2033. Services are also growing, moving from $138.60 million to $242.05 million in the same period, emphasizing the increasing trend towards comprehensive quality control solutions.
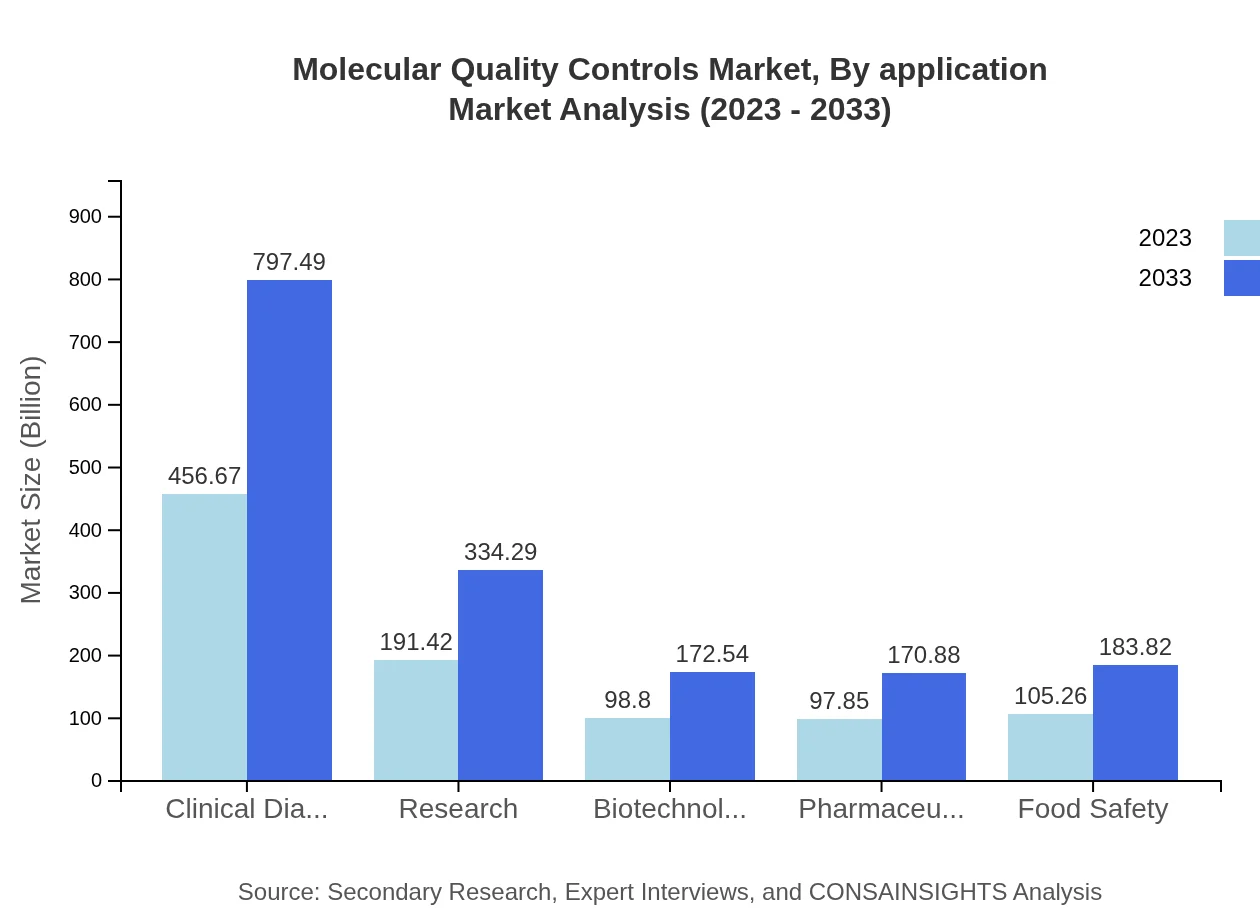
Molecular Quality Controls Market Analysis By Application
The applications of Molecular Quality Controls are primarily observed in clinical diagnostics, research, and quality assurance in biotechnology and pharmaceuticals. Clinical diagnostics holds a significant segment, accounting for $456.67 million in 2023 and expected to reach $797.49 million by 2033. Research applications are projected to grow from $191.42 million to $334.29 million, reflecting a strong demand for quality assurance in R&D.
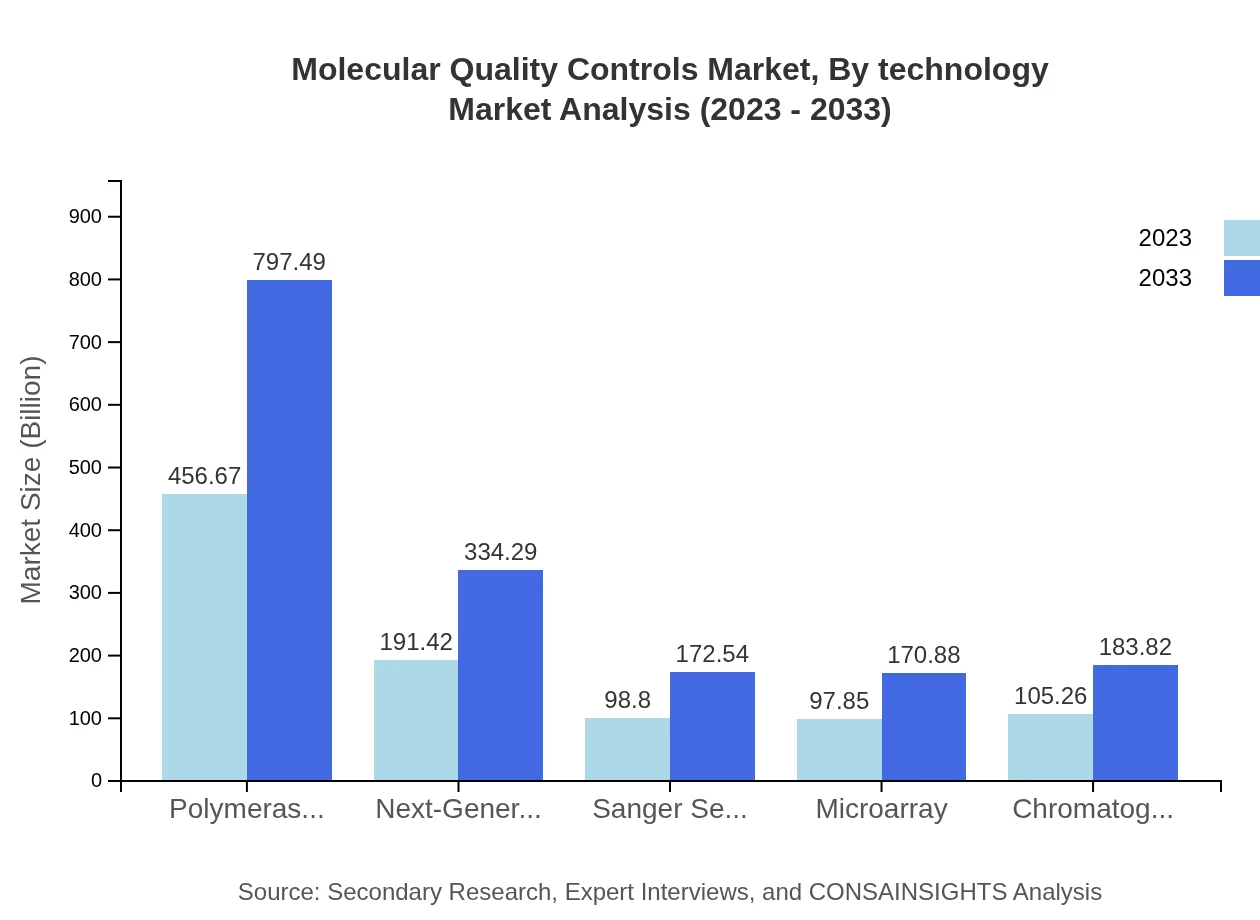
Molecular Quality Controls Market Analysis By Technology
Technologies employed in the Molecular Quality Controls market include PCR, NG sequencing, and microarrays. PCR technology is expected to remain a front-runner, with its market share being $456.67 million in 2023 and rising to $797.49 million by 2033. NGS is also on a growth trajectory, moving from $191.42 million to $334.29 million, making it a key player in diagnostic advancements.
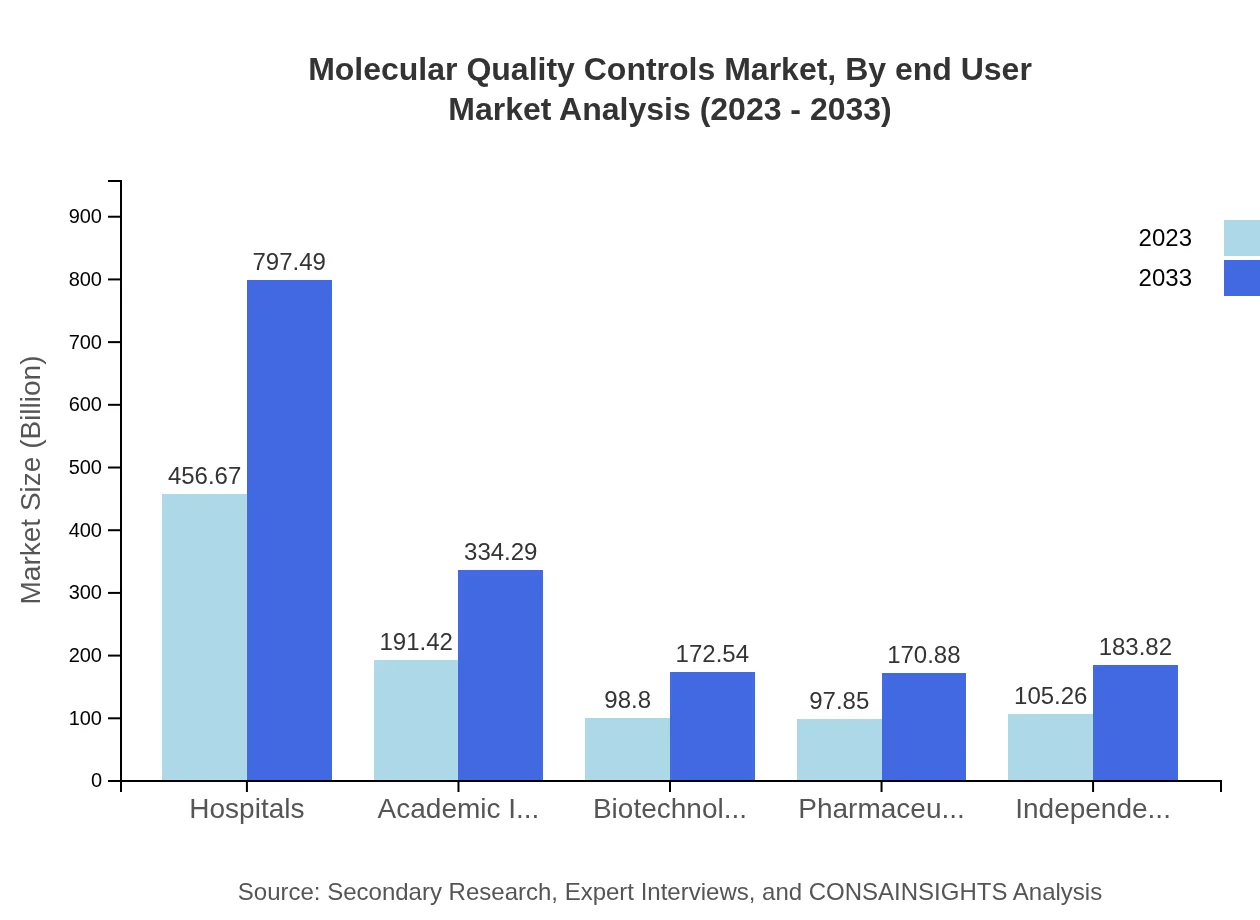
Molecular Quality Controls Market Analysis By End User
End-users of Molecular Quality Controls include hospitals, academic institutions, biotechnology firms, and pharmaceuticals. Hospitals represent the largest segment at $456.67 million in 2023, anticipated to reach $797.49 million by 2033. Academic institutions are also growing significantly, from $191.42 million to $334.29 million, as research efforts intensify globally.
Molecular Quality Controls Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Molecular Quality Controls Industry
Thermo Fisher Scientific:
Thermo Fisher Scientific is a leading company in the Molecular Quality Controls market, offering a wide range of reagents and quality control assays for diagnostics and research.Roche Diagnostics:
Roche Diagnostics specializes in molecular testing and provides robust quality control solutions that enhance the reliability of molecular results.Bio-Rad Laboratories:
Bio-Rad Laboratories offers innovative quality control products and reagents essential for precise molecular assay performance.Abbott Laboratories:
Abbott Laboratories is a key player in the molecular diagnostics space, providing advanced quality controls and diagnostic products.Merck Group:
Merck Group supplies essential reagents and automated solutions that streamline quality control processes for molecular diagnostics.We're grateful to work with incredible clients.









FAQs
What is the market size of molecular Quality Controls?
The global molecular quality controls market is valued at approximately $950 million in 2023, with a projected CAGR of 5.6%, reaching an estimated market size in 2033 that reflects significant growth.
What are the key market players or companies in this molecular Quality Controls industry?
Key players in the molecular quality controls industry include major companies that specialize in reagents and laboratory supplies, biotechnology firms, and diagnostics manufacturers, working collaboratively in R&D and quality assurance.
What are the primary factors driving the growth in the molecular Quality Controls industry?
Growth in molecular quality controls is primarily driven by increasing demand for accurate and reliable diagnostic tests, the expansion of clinical laboratories, advancements in biotechnology, and rising investments in R&D from pharmaceutical companies.
Which region is the fastest Growing in the molecular quality controls?
North America is the fastest-growing region in the molecular quality controls market, projected to grow from $355.30 million in 2023 to $620.47 million by 2033, significantly outpacing other regions.
Does ConsaInsights provide customized market report data for the molecular quality controls industry?
Yes, ConsaInsights offers customized market report data tailored to the molecular quality controls industry, allowing clients to specify particular areas of interest and obtain detailed insights.
What deliverables can I expect from this molecular Quality Controls market research project?
Deliverables include a comprehensive market report featuring detailed analysis, regional breakdown, segment insights, market trends, and projections, ensuring a holistic understanding of the molecular quality controls landscape.
What are the market trends of molecular quality controls?
Current trends in the molecular quality controls market include a rising preference for automation in laboratories, integration of AI and machine learning in diagnostics, and growing focus on personalized medicine and precision diagnostics.