Monoclonal Antibody Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: monoclonal-antibody-therapeutics
Monoclonal Antibody Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This detailed market report covers the Monoclonal Antibody Therapeutics landscape, providing insights on market size, growth forecasts, and industry analysis for the period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
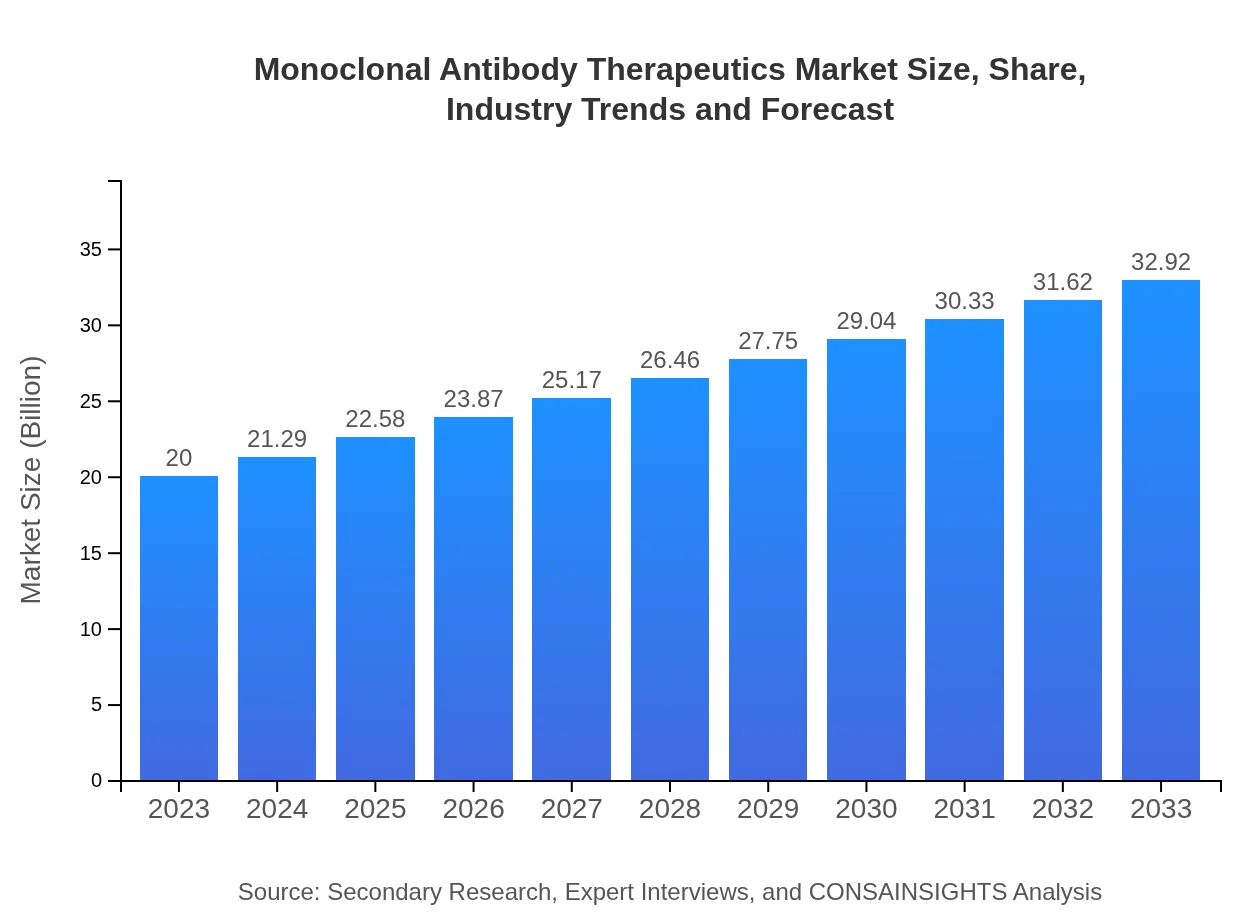
| 2023 Market Size | $20.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $32.92 Billion |
| Top Companies | Roche, Amgen, Absci, Merck & Co., Johnson & Johnson |
| Last Modified Date | 31 January 2026 |
Monoclonal Antibody Therapeutics Market Overview
Customize Monoclonal Antibody Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Monoclonal Antibody Therapeutics market size, growth, and forecasts.
- ✔ Understand Monoclonal Antibody Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Monoclonal Antibody Therapeutics
What is the Market Size & CAGR of Monoclonal Antibody Therapeutics market in 2033?
Monoclonal Antibody Therapeutics Industry Analysis
Monoclonal Antibody Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Monoclonal Antibody Therapeutics Market Analysis Report by Region
Europe Monoclonal Antibody Therapeutics Market Report:
In 2023, Europe accounted for $5.16 billion in the market, with a projected increase to $8.49 billion by 2033. Growing R&D investment and favorable reimbursement policies in healthcare are key growth factors.Asia Pacific Monoclonal Antibody Therapeutics Market Report:
In 2023, the Asia Pacific Monoclonal Antibody Therapeutics market was valued at $4.24 billion, projected to grow to $6.97 billion by 2033. This growth is driven by increasing investments in healthcare infrastructure and rising healthcare expenditure.North America Monoclonal Antibody Therapeutics Market Report:
North America dominated the market with an estimated size of $6.70 billion in 2023, forecasted to grow to $11.03 billion by 2033. Presence of major pharmaceutical companies and advanced healthcare systems drive this growth.South America Monoclonal Antibody Therapeutics Market Report:
The South American market was worth $1.18 billion in 2023 and is expected to reach $1.95 billion by 2033. Rising incidences of chronic diseases and expanding access to healthcare contribute to this growth.Middle East & Africa Monoclonal Antibody Therapeutics Market Report:
The Middle East and Africa market was valued at $2.72 billion in 2023 and is expected to grow to $4.47 billion by 2033. The region is witnessing increased focus on improving healthcare accessibility and efficacy.Tell us your focus area and get a customized research report.
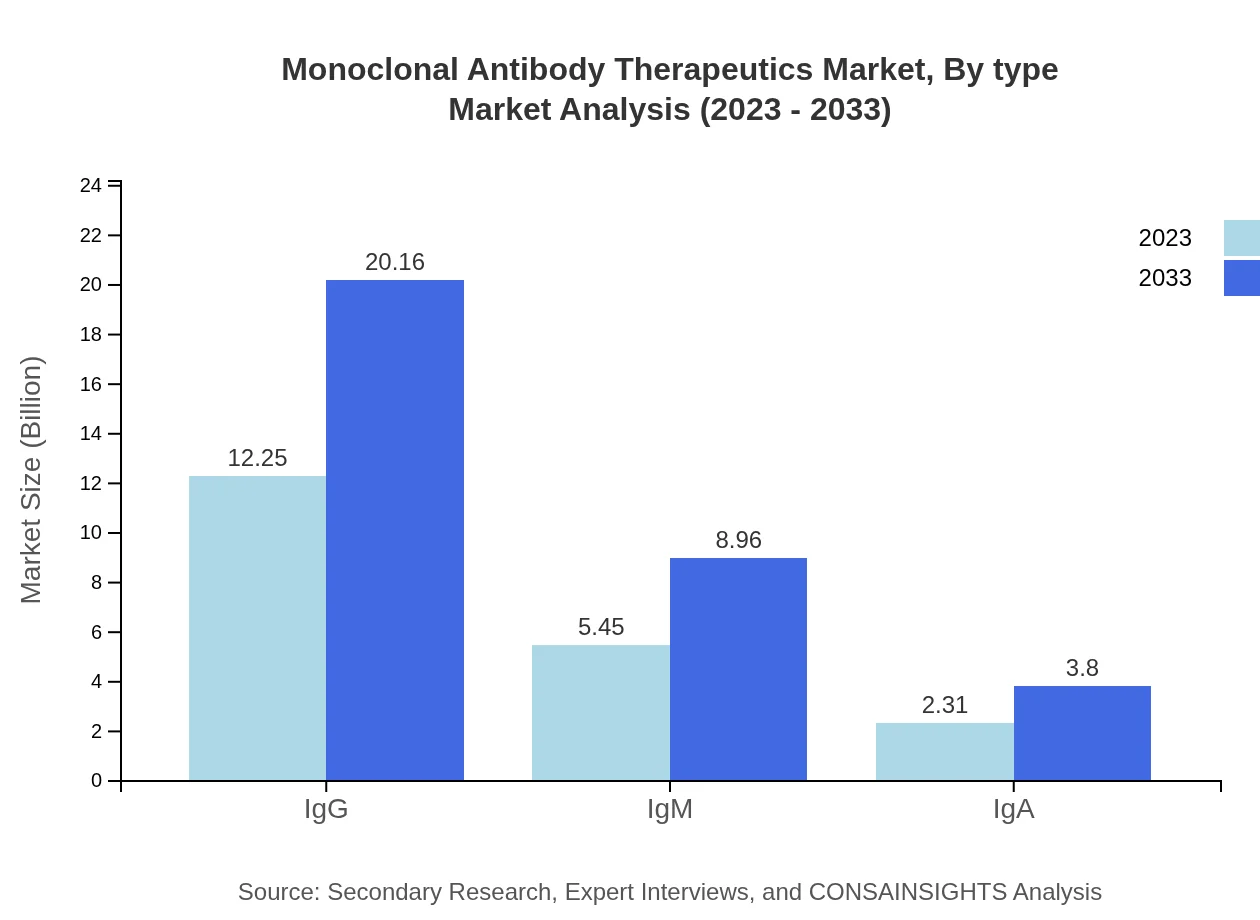
Monoclonal Antibody Therapeutics Market Analysis By Type
The market by type is led by IgG antibodies, which accounted for $12.25 billion in 2023 and are projected to reach $20.16 billion by 2033. IgM and IgA antibodies also contribute significantly, serving specialized therapeutic roles.
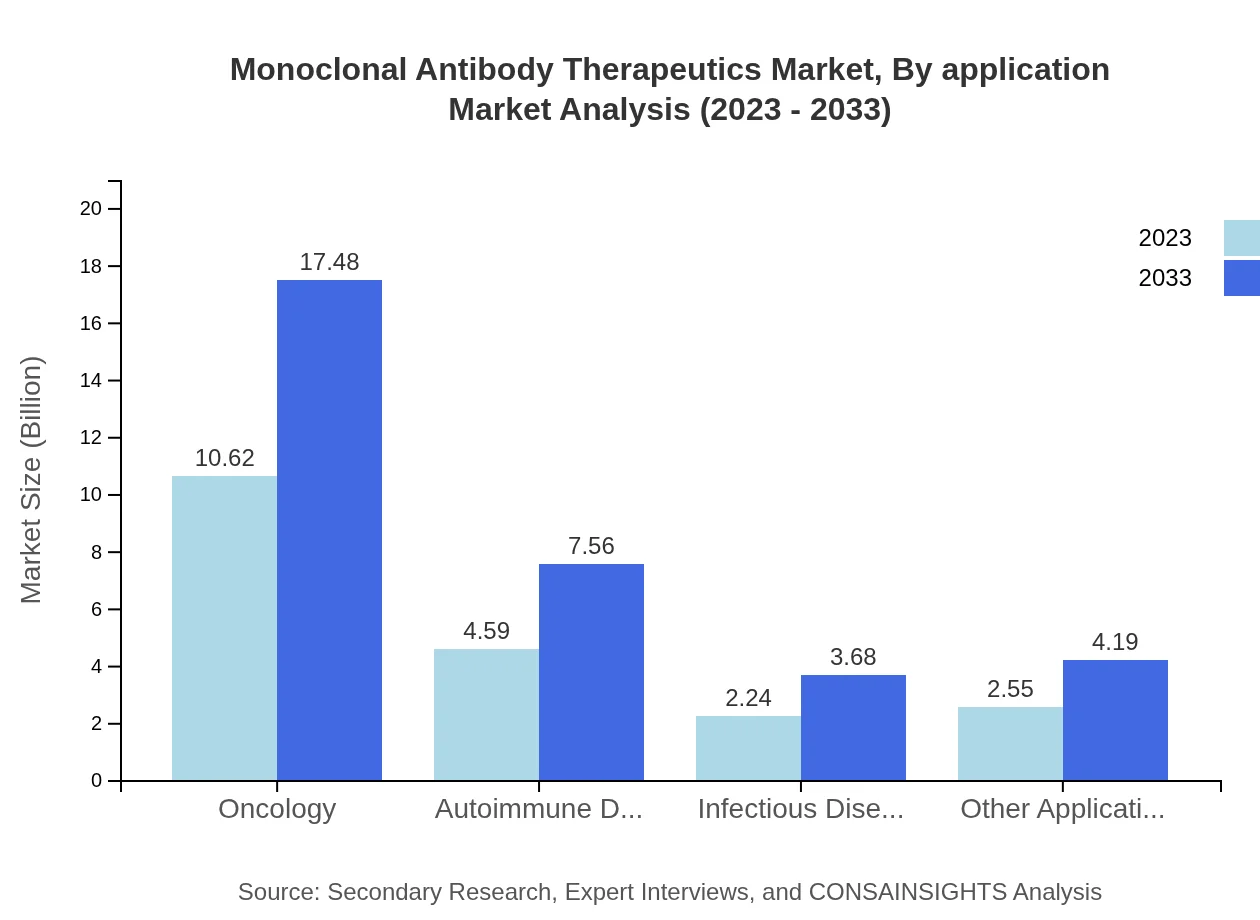
Monoclonal Antibody Therapeutics Market Analysis By Application
Oncology is the largest application, estimated at $10.62 billion in 2023 and expected to reach $17.48 billion in 2033. Other applications such as autoimmune disorders ($4.59 billion in 2023) and infectious diseases ($2.24 billion) show robust growth as well.
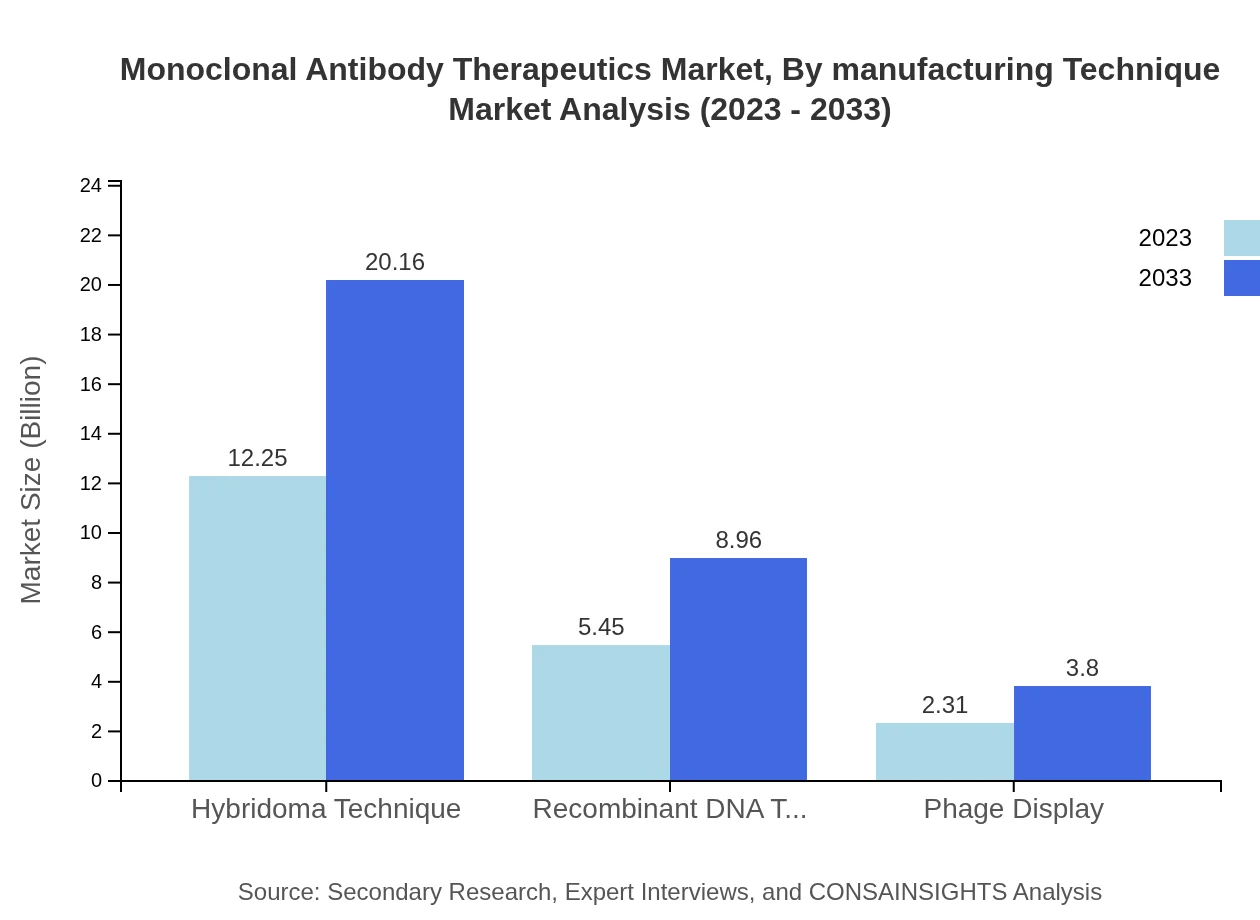
Monoclonal Antibody Therapeutics Market Analysis By Manufacturing Technique
The Hybridoma Technique dominates the manufacturing technique segment, projected to grow from $12.25 billion in 2023 to $20.16 billion by 2033. Other techniques, such as Recombinant DNA Technology and Phage Display, are also gaining traction.
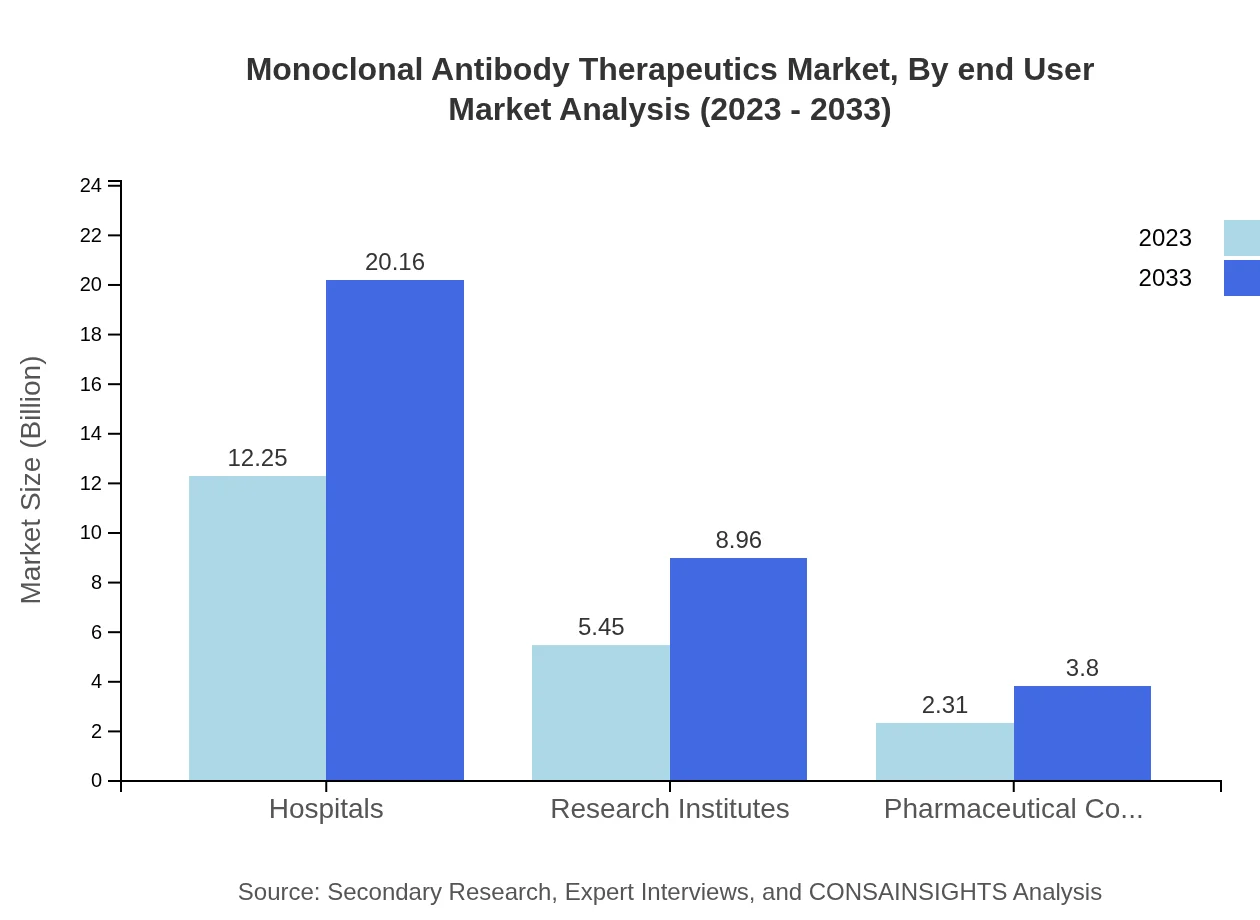
Monoclonal Antibody Therapeutics Market Analysis By End User
Hospitals represent the largest end-user segment, valued at $12.25 billion in 2023, rising to $20.16 billion by 2033. Research institutes and pharmaceutical companies are crucial for facilitating R&D and commercialization of new therapies, contributing $5.45 billion and $2.31 billion, respectively.
Monoclonal Antibody Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Monoclonal Antibody Therapeutics Industry
Roche:
A pioneer in biologics, Roche leads the market with a strong portfolio of monoclonal antibody drugs targeting multiple diseases, especially in oncology and immunology.Amgen:
Amgen specializes in innovative biopharmaceuticals and offers a diverse range of monoclonal antibody therapies, with a focus on improving patient outcomes.Absci:
Known for its proprietary deep learning technology, Absci develops next-generation therapeutic monoclonal antibodies for diverse indications, enhancing discovery capabilities.Merck & Co.:
Merck has a significant presence in the monoclonal antibody market, with leading products that have transformed the treatment landscape for cancer and infectious diseases.Johnson & Johnson:
Johnson & Johnson's Janssen Pharmaceuticals has a robust portfolio of monoclonal antibodies targeting a variety of therapeutic areas, continuously contributing to innovation.We're grateful to work with incredible clients.









FAQs
What is the market size of monoclonal Antibody Therapeutics?
The monoclonal-antibody-therapeutics market is projected to reach a size of $20 billion by 2033, demonstrating a compound annual growth rate (CAGR) of 5% from 2023 to 2033.
What are the key market players or companies in this monoclonal Antibody Therapeutics industry?
Key players in the monoclonal-antibody-therapeutics industry typically include major biopharmaceutical companies committed to oncology, autoimmune diseases, and infectious diseases. These companies leverage advanced technologies and significant investment in research and development.
What are the primary factors driving the growth in the monoclonal Antibody Therapeutics industry?
The growth in the monoclonal-antibody-therapeutics industry is driven by increasing prevalence of chronic diseases, advancements in biotechnology, improved treatment outcomes, and a growing aging population, alongside significant government and private funding for innovative therapies.
Which region is the fastest Growing in the monoclonal Antibody Therapeutics?
The North American region is currently the fastest-growing market for monoclonal-antibody-therapeutics, expected to grow from $6.70 billion in 2023 to $11.03 billion by 2033, reflecting a rise in healthcare investments and demand for innovative treatments.
Does ConsaInsights provide customized market report data for the monoclonal Antibody Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs within the monoclonal-antibody-therapeutics industry, allowing for focused insights on unique market segments and geographical areas.
What deliverables can I expect from this monoclonal Antibody Therapeutics market research project?
Deliverables for the monoclonal-antibody-therapeutics market research project include detailed market analysis reports, segmentation data, competitive landscape assessments, and future market projections to aid in strategic decision-making.
What are the market trends of monoclonal Antibody Therapeutics?
Current market trends for monoclonal-antibody-therapeutics include increased adoption of combination therapies, a focus on personalized medicine, and innovation in antibody design, alongside rising investments in biosimilars and targeted therapies.