Mononucleosis Diagnostic Market Report
Published Date: 31 January 2026 | Report Code: mononucleosis-diagnostic
Mononucleosis Diagnostic Market Size, Share, Industry Trends and Forecast to 2033
This report presents a comprehensive analysis of the Mononucleosis Diagnostic market, detailing current trends, market size, projections up to 2033, and competitive dynamics across regions and segments.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
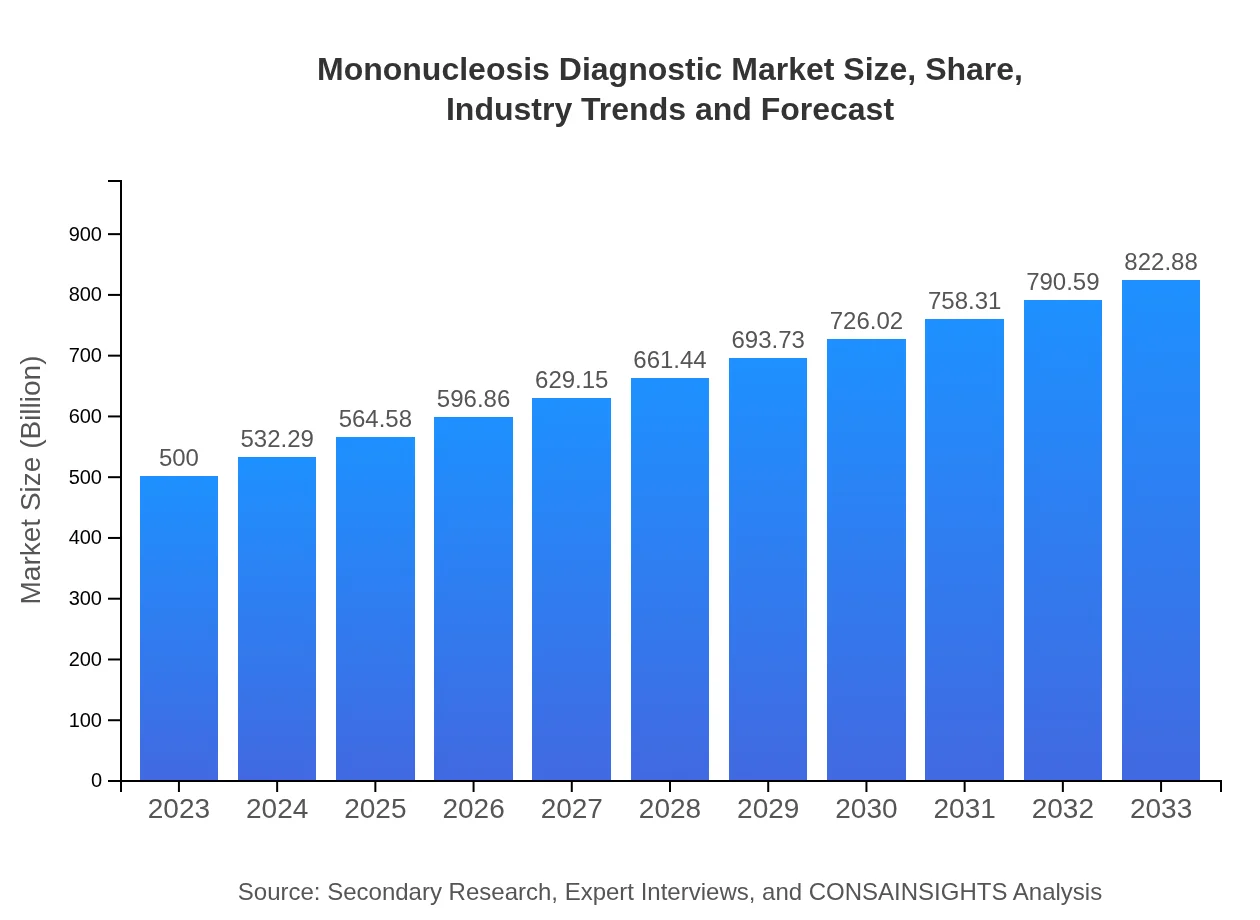
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $822.88 Million |
| Top Companies | Abbott Laboratories, Thermo Fisher Scientific, Roche Diagnostics, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Mononucleosis Diagnostic Market Overview
Customize Mononucleosis Diagnostic Market Report market research report
- ✔ Get in-depth analysis of Mononucleosis Diagnostic market size, growth, and forecasts.
- ✔ Understand Mononucleosis Diagnostic's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Mononucleosis Diagnostic
What is the Market Size & CAGR of Mononucleosis Diagnostic market in 2023?
Mononucleosis Diagnostic Industry Analysis
Mononucleosis Diagnostic Market Segmentation and Scope
Tell us your focus area and get a customized research report.
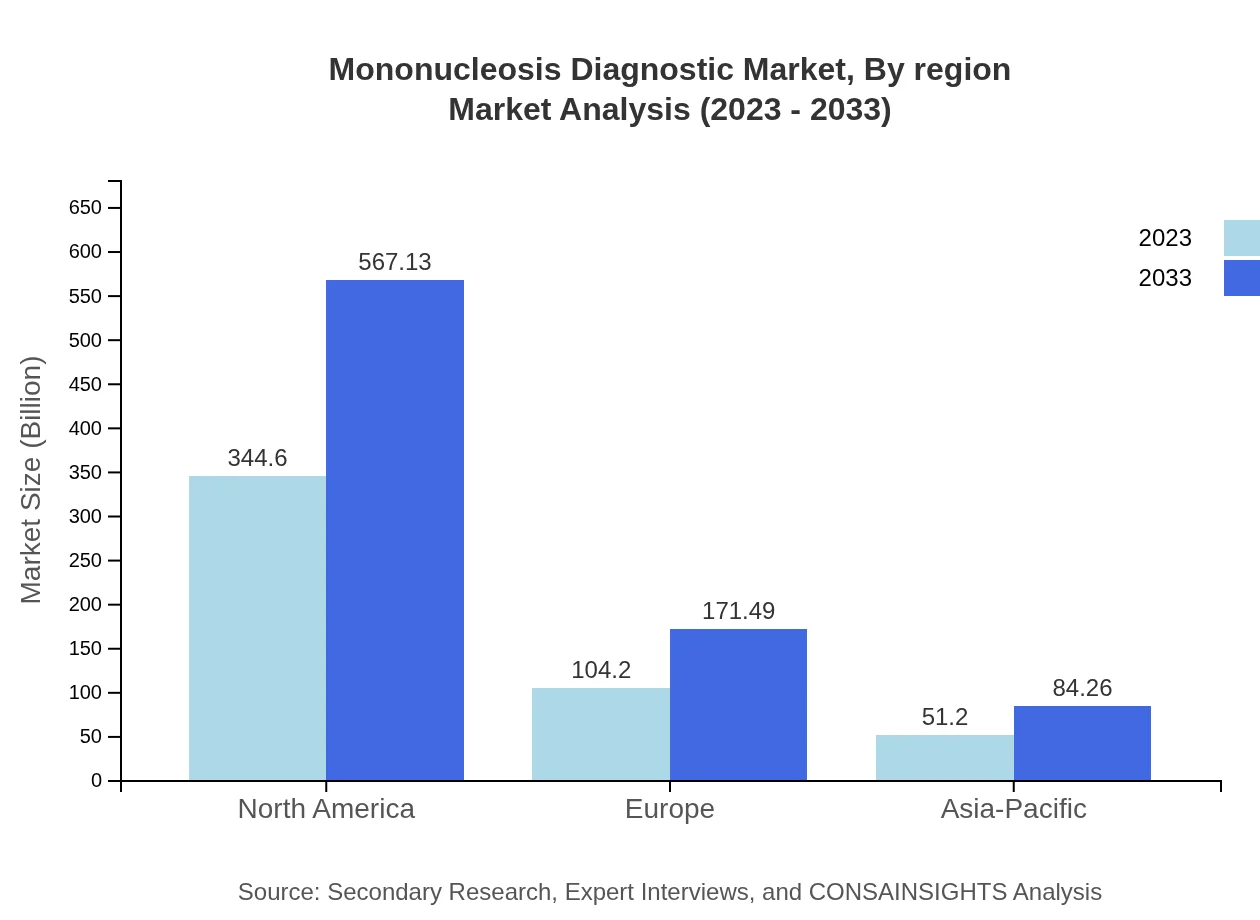
Mononucleosis Diagnostic Market Analysis Report by Region
Europe Mononucleosis Diagnostic Market Report:
Europe's market is anticipated to increase from $133.95 million in 2023, achieving $220.45 million by 2033, thanks to strong healthcare policies, rising focus on infectious disease management, and technological advancements in diagnostic testing.Asia Pacific Mononucleosis Diagnostic Market Report:
In the Asia Pacific region, the Mononucleosis Diagnostic market is expected to grow from $96.50 million in 2023 to $158.82 million by 2033, reflecting a growing emphasis on healthcare infrastructure and rising preventive healthcare measures. Increasing healthcare spending and awareness of EBV are pivotal in driving this growth.North America Mononucleosis Diagnostic Market Report:
North America will lead the market, growing from $160.80 million in 2023 to approximately $264.64 million by 2033. This robust growth can be attributed to advanced healthcare technologies, a high prevalence of mononucleosis among youngsters, and increased funding for research.South America Mononucleosis Diagnostic Market Report:
The South American market, valued at $45.20 million in 2023 and reaching $74.39 million by 2033, shows steady growth. This trend is driven by improvements in healthcare systems, coupled with high rates of infectious diseases, prompting the need for better diagnostics and treatments.Middle East & Africa Mononucleosis Diagnostic Market Report:
The Middle East and Africa region is projected to grow from a market size of $63.55 million in 2023 to $104.59 million in 2033. This growth is spurred by enhanced healthcare access, boosted diagnostic capabilities, and increased awareness surrounding mononucleosis.Tell us your focus area and get a customized research report.
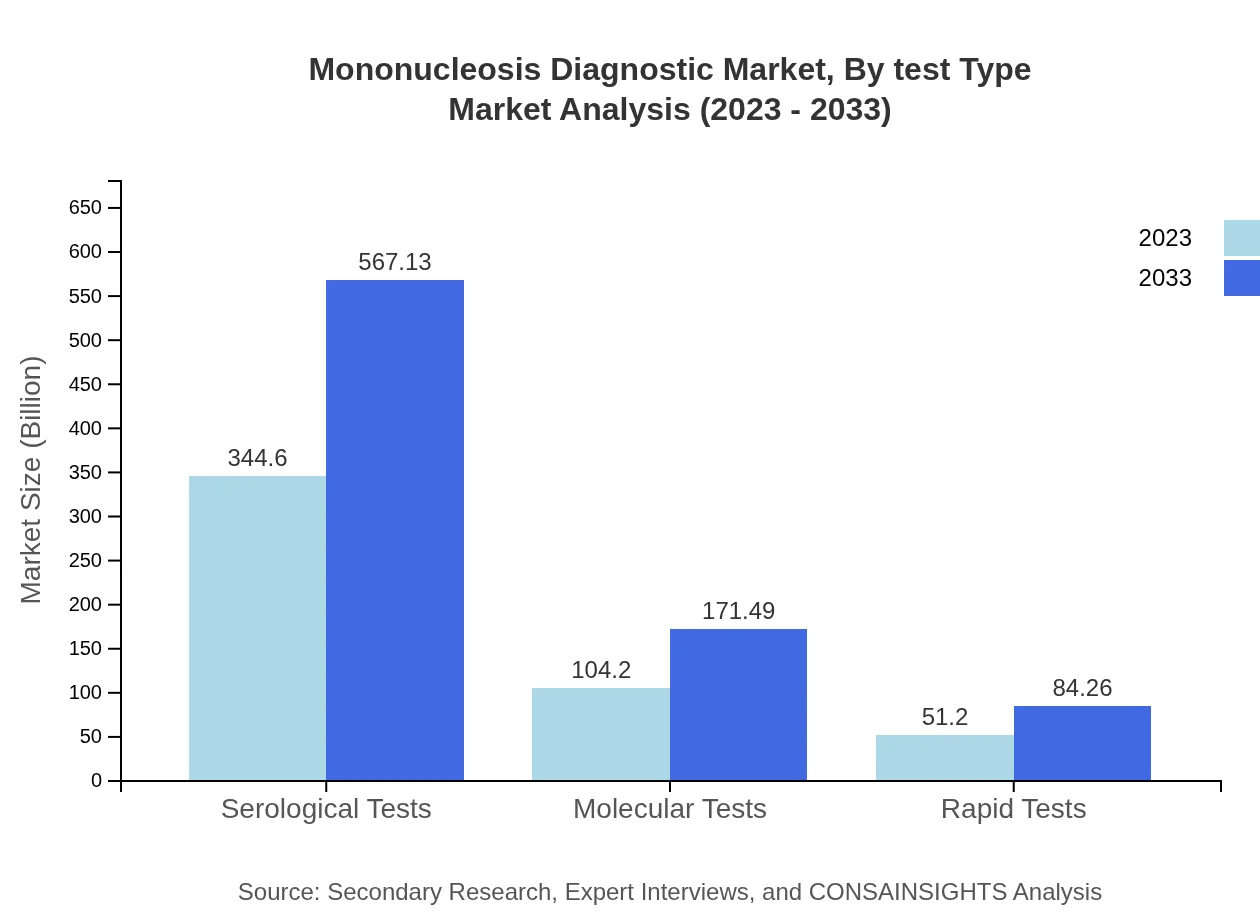
Mononucleosis Diagnostic Market Analysis By Test Type
The Mononucleosis diagnostic market by test type reveals a dominant share for serological tests, accounting for approximately 68.92% of the market in 2023, growing in tandem with molecular testing methods, which exhibit increasing adoption due to their higher specificity and speed.
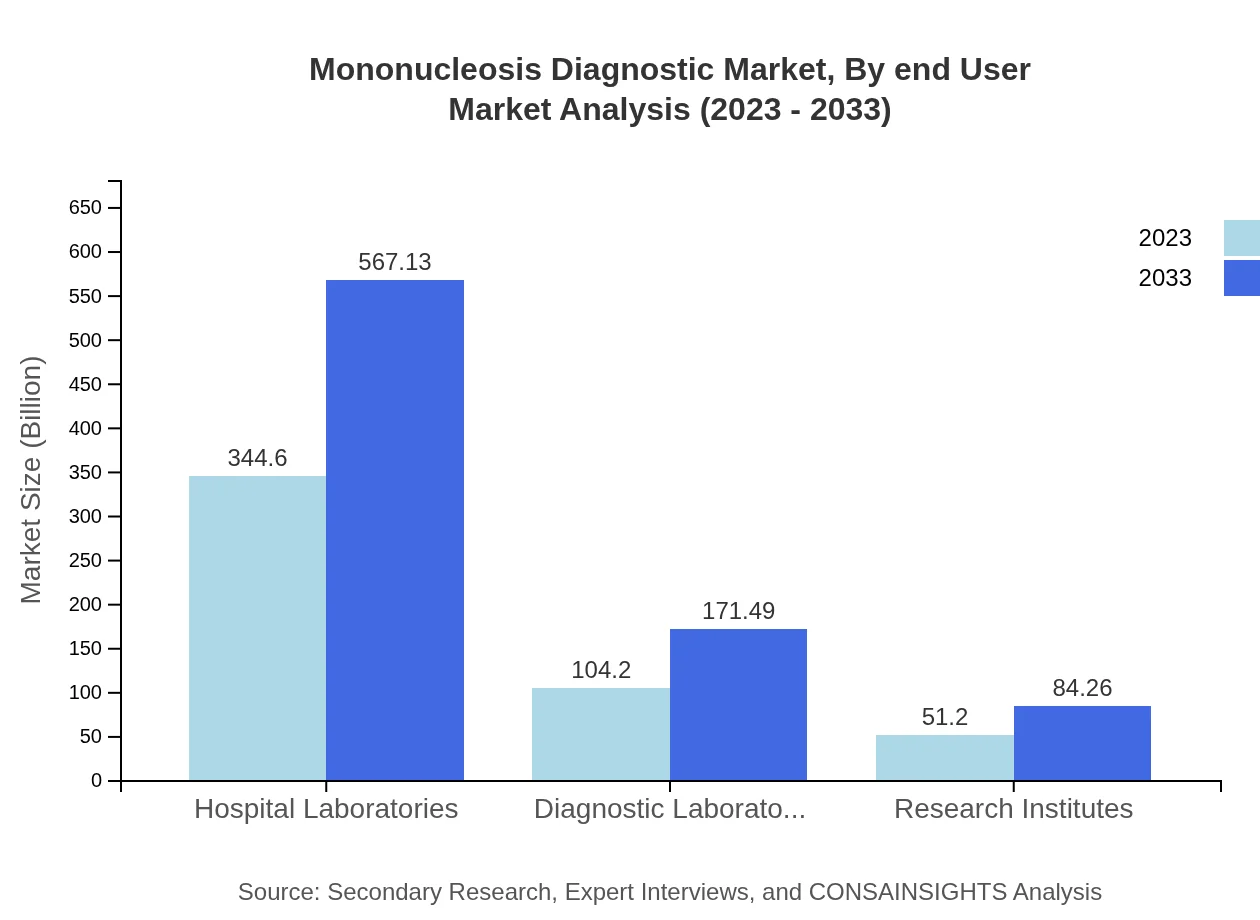
Mononucleosis Diagnostic Market Analysis By End User
Hospital laboratories are the largest segment, expected to maintain a share of around 68.92% throughout the forecast period, owing to their critical role in diagnosis and patient management, alongside a rising demand for rapid testing.
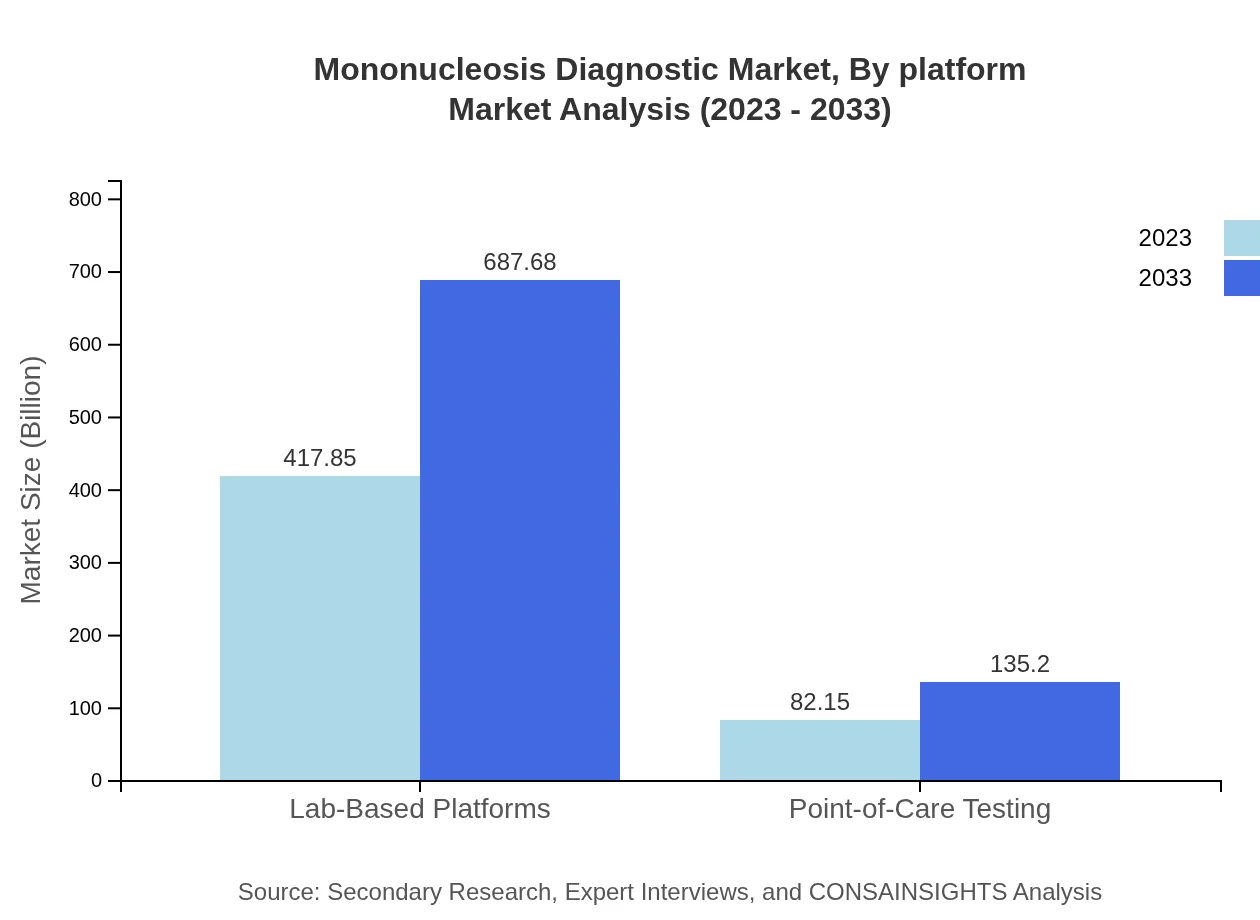
Mononucleosis Diagnostic Market Analysis By Platform
Lab-based platforms lead the market, projected to account for over 83.57% of the market share in 2023 due to their widespread use in clinical diagnostics, complemented by growing demand for point-of-care testing products to enhance accessibility.
Mononucleosis Diagnostic Market Analysis By Region
Each geographical market displays unique growth patterns influenced by local healthcare undemand, technological advancements, and regulatory policies, indicating varied strategies by key players in each region.
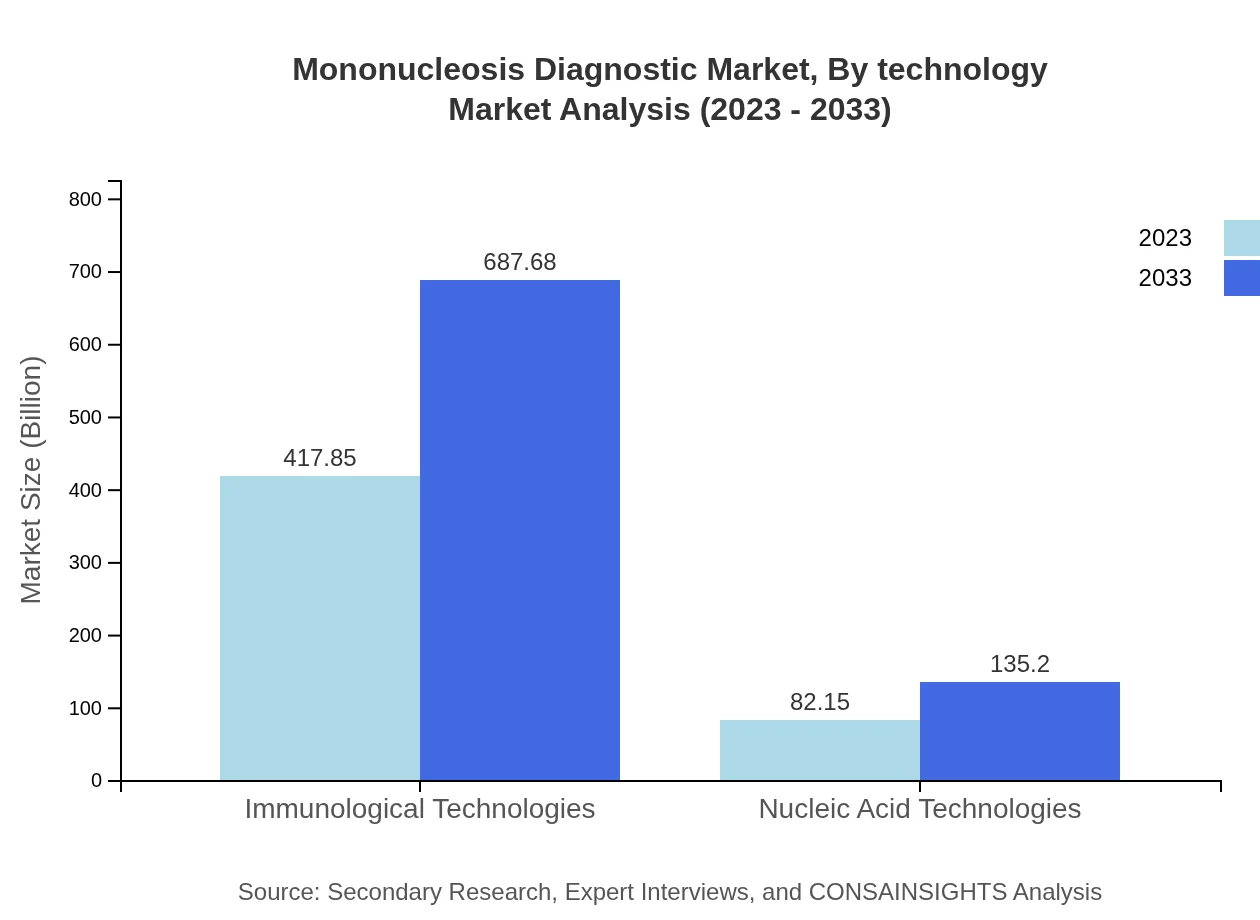
Mononucleosis Diagnostic Market Analysis By Technology
Immunological technologies are set to dominate with over 83.57% market share in 2023, driven by their robust performance in serological testing, while nucleic acid technologies are gaining traction.
Mononucleosis Diagnostic Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Mononucleosis Diagnostic Industry
Abbott Laboratories:
A global healthcare company that offers a broad range of diagnostic solutions including innovative tests for mononucleosis.Thermo Fisher Scientific:
Known for its advanced laboratory equipment and diagnostic kits, it plays a pivotal role in the development of diagnostic tests for infectious diseases including mononucleosis.Roche Diagnostics:
A leading player in the diagnostics industry, providing extensive testing solutions, including serological and molecular tests for mononucleosis.Siemens Healthineers:
Specializes in diagnostic imaging and lab diagnostics, contributing significant innovations for infectious disease testing globally.We're grateful to work with incredible clients.









FAQs
What is the market size of Mononucleosis Diagnostic?
The Mononucleosis Diagnostic market is valued at approximately $500 million in 2023, with a projected CAGR of 5% over the next decade, indicating robust growth potential in an evolving healthcare landscape.
What are the key market players or companies in this Mononucleosis Diagnostic industry?
Key players in the Mononucleosis Diagnostic industry include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific, providing innovative testing solutions and technologies in digital diagnostics.
What are the primary factors driving the growth in the Mononucleosis Diagnostic industry?
Growth in the Mononucleosis Diagnostic market is driven by increasing prevalence of infectious diseases, technological advancements in diagnostic testing, and growing awareness of early diagnosis among healthcare providers and patients.
Which region is the fastest Growing in the Mononucleosis Diagnostic?
The fastest-growing region in the Mononucleosis Diagnostic market is North America, expected to rise from $160.80 million in 2023 to $264.64 million by 2033, reflecting increased healthcare spending and technological improvements.
Does ConsaInsights provide customized market report data for the Mononucleosis Diagnostic industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the Mononucleosis Diagnostic industry, providing in-depth analysis that aligns with client requirements for strategic decision-making.
What deliverables can I expect from this Mononucleosis Diagnostic market research project?
Clients can expect comprehensive deliverables including detailed market analysis, segmentation breakdowns by region and type, competitor analysis, as well as strategic recommendations to enhance market positioning.
What are the market trends of Mononucleosis Diagnostic?
Trends in the Mononucleosis Diagnostic market include a rising focus on point-of-care testing, advancements in lab-based platforms, and a shift towards molecular and serological testing for accuracy and efficiency.