Mri Compatible Iv Infusion Pumps Market Report
Published Date: 31 January 2026 | Report Code: mri-compatible-iv-infusion-pumps
Mri Compatible Iv Infusion Pumps Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the MRI Compatible IV Infusion Pumps market, highlighting trends, growth prospects, and key players from 2023 to 2033. Insights include market size, segmentation, technological advancements, and regional performance to aid stakeholders in informed decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
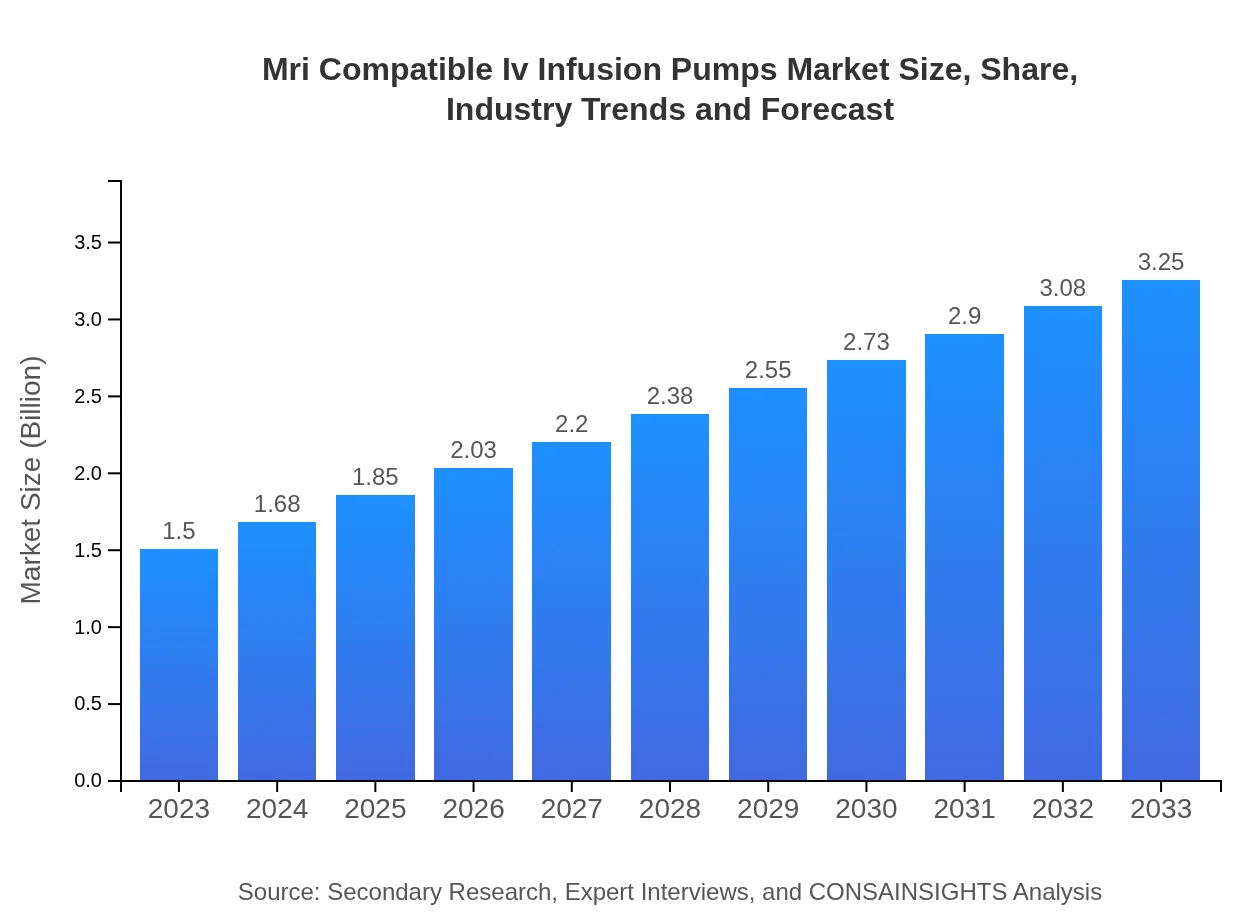
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $3.25 Billion |
| Top Companies | B. Braun Melsungen AG, Fresenius Kabi AG, Smiths Medical, CareFusion (Becton, Dickinson and Company), Medtronic |
| Last Modified Date | 31 January 2026 |
MRI Compatible IV Infusion Pumps Market Overview
Customize Mri Compatible Iv Infusion Pumps Market Report market research report
- ✔ Get in-depth analysis of Mri Compatible Iv Infusion Pumps market size, growth, and forecasts.
- ✔ Understand Mri Compatible Iv Infusion Pumps's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Mri Compatible Iv Infusion Pumps
What is the Market Size & CAGR of MRI Compatible IV Infusion Pumps market in 2023?
MRI Compatible IV Infusion Pumps Industry Analysis
MRI Compatible IV Infusion Pumps Market Segmentation and Scope
Tell us your focus area and get a customized research report.
MRI Compatible IV Infusion Pumps Market Analysis Report by Region
Europe Mri Compatible Iv Infusion Pumps Market Report:
In Europe, the market for MRI compatible IV infusion pumps was valued at $0.41 billion in 2023 and is expected to reach $0.89 billion by 2033. The market dynamics include a strong focus on improved healthcare delivery systems and government initiatives aimed at funding healthcare innovations.Asia Pacific Mri Compatible Iv Infusion Pumps Market Report:
In the Asia-Pacific region, the MRI compatible IV infusion pumps market was valued at $0.31 billion in 2023 and is projected to grow to $0.66 billion by 2033. This growth is driven by increasing healthcare investments, expanding healthcare infrastructure, and the rising prevalence of diseases requiring infusion therapies.North America Mri Compatible Iv Infusion Pumps Market Report:
North America remains a leader in the MRI compatible IV infusion pumps market, with an initial valuation of $0.54 billion in 2023 poised to rise to $1.17 billion by 2033. The robust growth is primarily due to technological innovations, high healthcare spending, and a strong regulatory framework promoting safety in medical devices.South America Mri Compatible Iv Infusion Pumps Market Report:
South America reflects moderate growth in the MRI compatible IV infusion pumps market, with a valuation of $0.10 billion in 2023 expected to reach $0.21 billion in 2033. The development is attributed to improving healthcare access and initiatives taken to modernize existing facilities.Middle East & Africa Mri Compatible Iv Infusion Pumps Market Report:
The Middle East and Africa show potential growth opportunities, with the market valued at $0.14 billion in 2023, projected to reach $0.31 billion by 2033. Increased healthcare investments and collaborations between public and private sectors are contributing to market advancements.Tell us your focus area and get a customized research report.
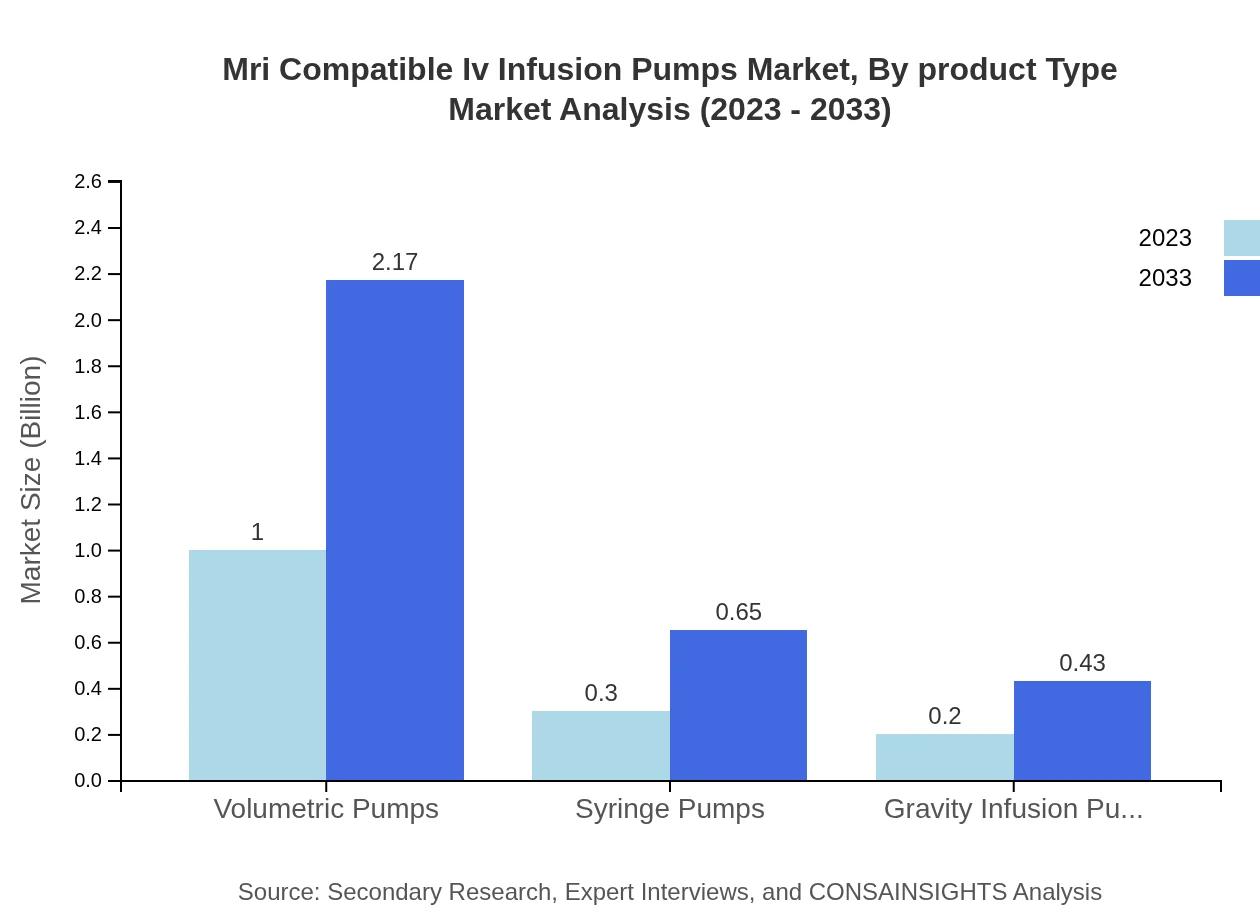
Mri Compatible Iv Infusion Pumps Market Analysis By Product Type
The market segmentation by product type focuses on microprocessor-controlled pumps, volumetric pumps, syringe pumps, and gravity-driven pumps. Microprocessor-controlled pumps dominate the space, accounting for approximately $1.00 billion in 2023 and expected to expand to $2.17 billion by 2033, driven by advanced drug delivery capabilities. Volumetric pumps and syringe pumps also maintain significant shares due to their versatility and ease of use in clinical environments.
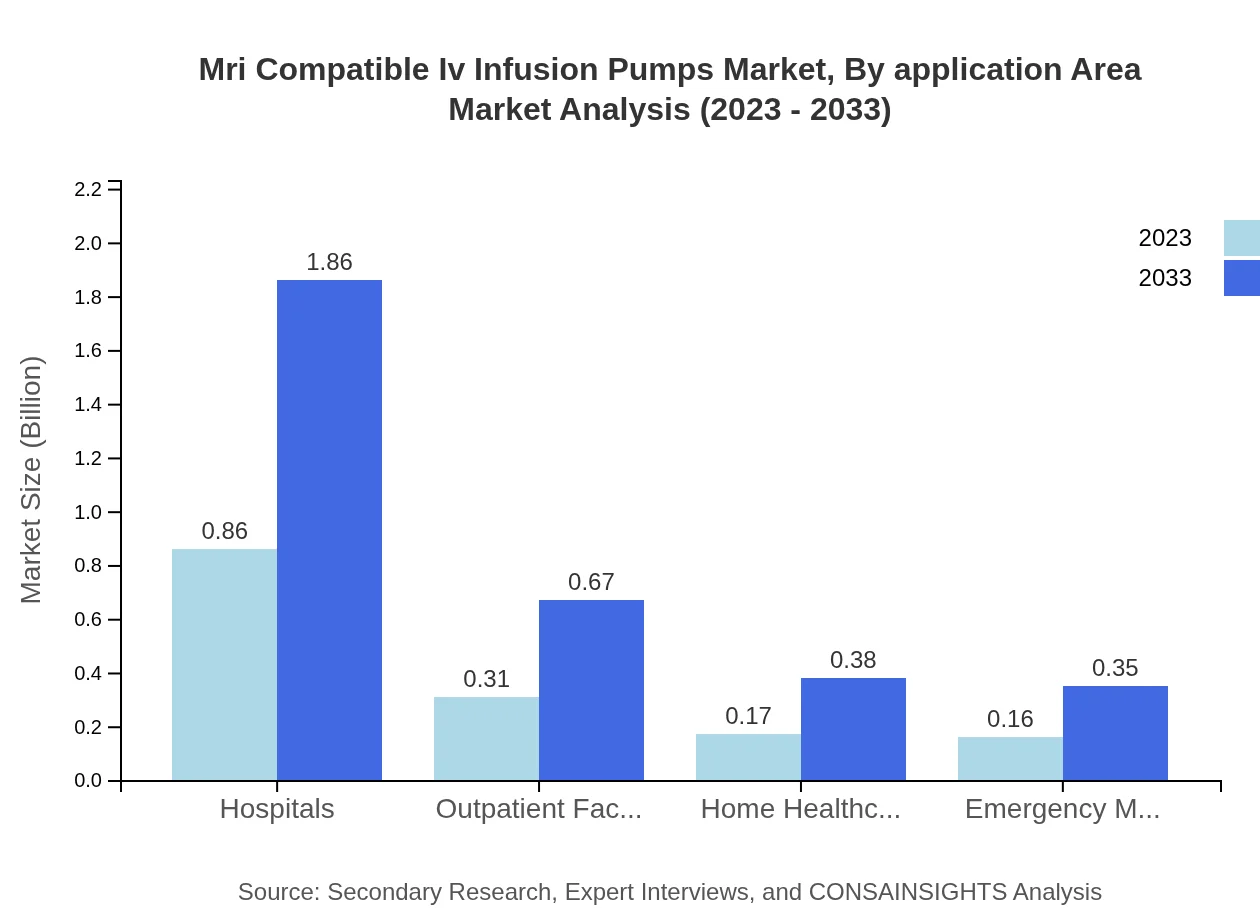
Mri Compatible Iv Infusion Pumps Market Analysis By Application Area
Within the application area, the MRI compatible IV infusion pumps serve hospitals, outpatient facilities, and home healthcare markets. Hospitals represent the largest application area, with a market size of $0.86 billion in 2023 anticipated to grow to $1.86 billion by 2033, reflecting the demand for reliable and precise drug delivery systems.
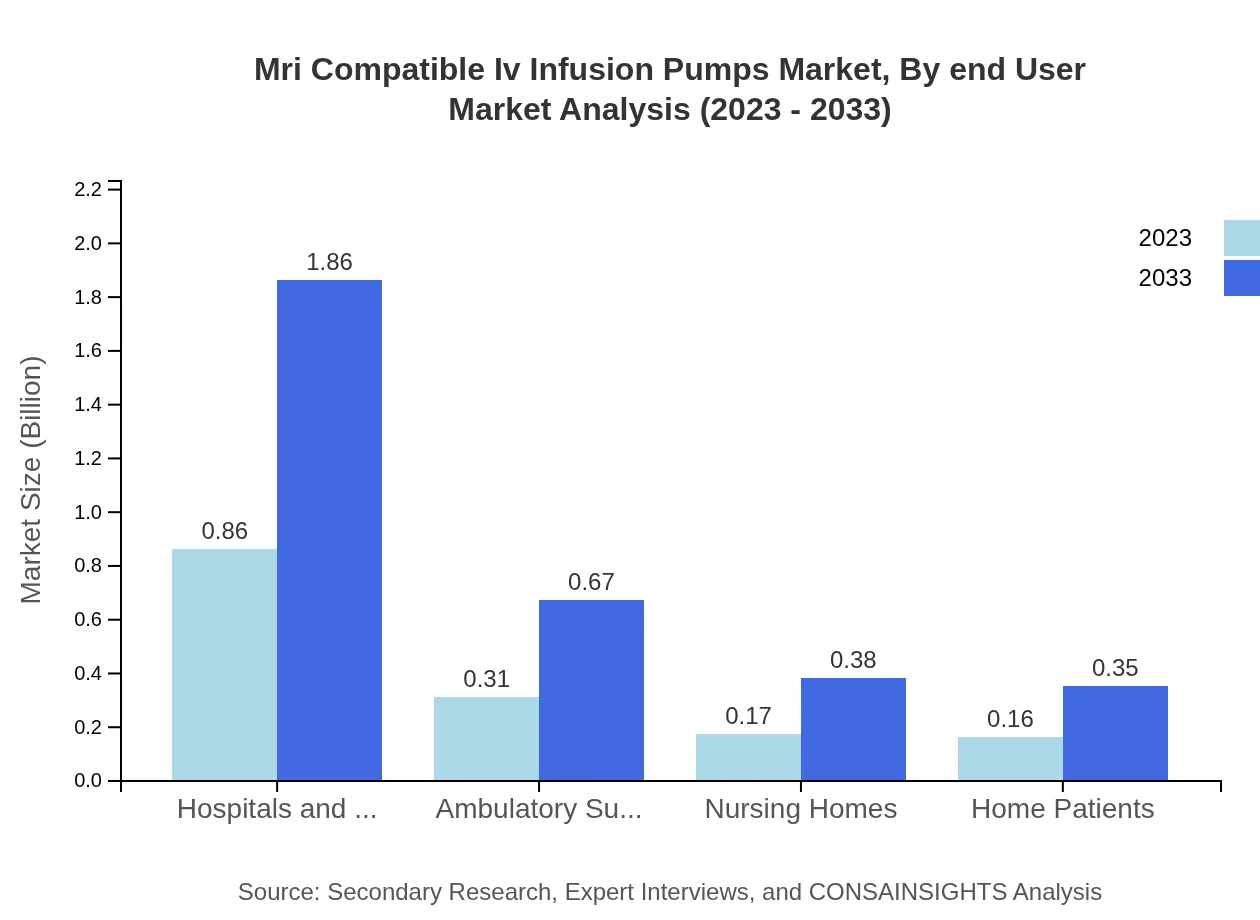
Mri Compatible Iv Infusion Pumps Market Analysis By End User
The end-user segmentation shows hospitals and clinics as the dominant segment, capturing 57.17% of the market share in 2023. Auxiliary segments include ambulatory surgical centers and nursing homes, with shares of 20.6% and 11.58% respectively. This distribution highlights the emphasis on safety and compliance across various healthcare settings.
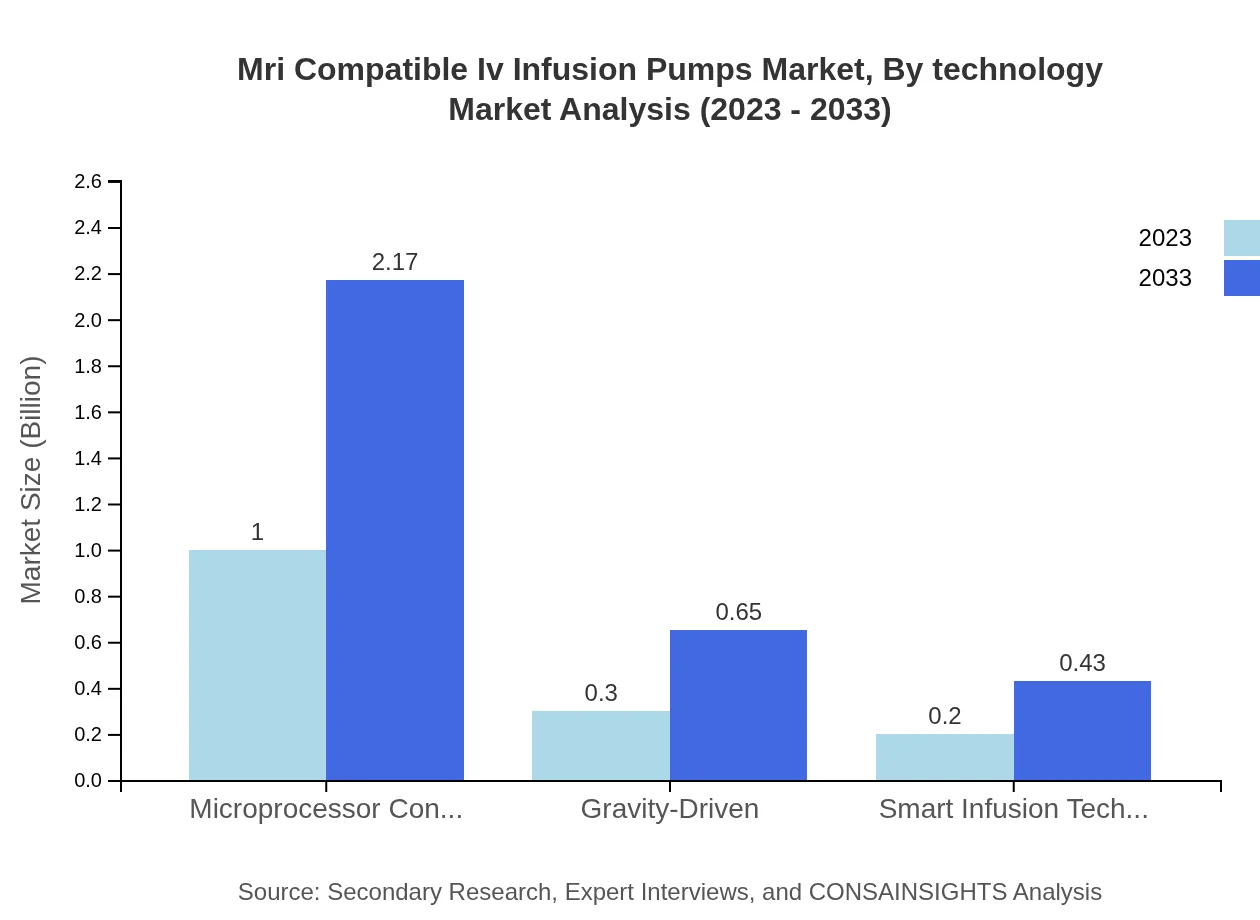
Mri Compatible Iv Infusion Pumps Market Analysis By Technology
The technology segment encompasses microprocessor-controlled, gravity-driven, and smart infusion technologies. The microprocessor-controlled technology holds the largest share at 66.74% in 2023, illustrating its pivotal role in enhancing infusion therapies' accuracy and efficiency.
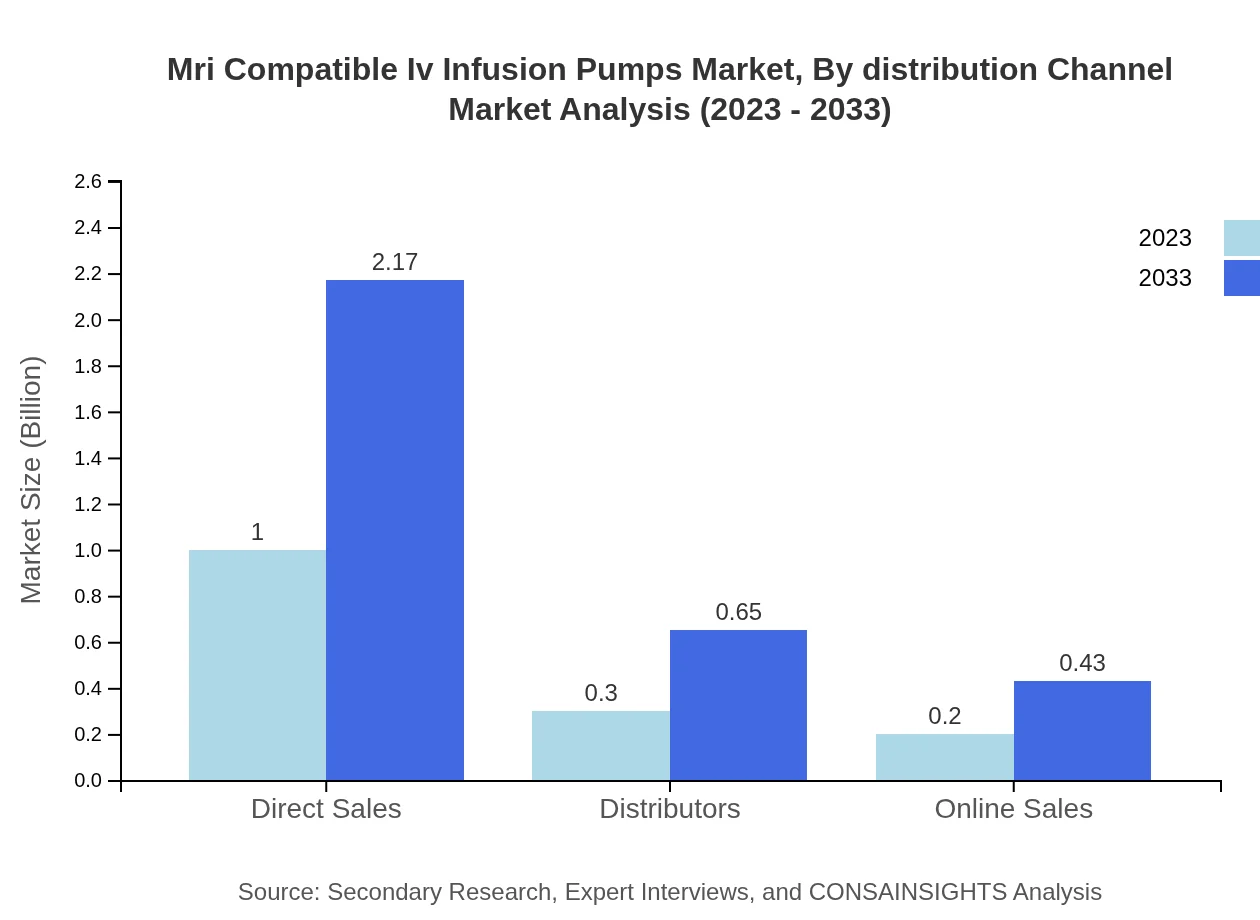
Mri Compatible Iv Infusion Pumps Market Analysis By Distribution Channel
Distribution channels for MRI compatible IV infusion pumps include direct sales, distributors, and online sales. Direct sales dominate with a market share of 66.74% in 2023, as they allow manufacturers to establish strong relationships with healthcare providers.
MRI Compatible IV Infusion Pumps Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in MRI Compatible IV Infusion Pumps Industry
B. Braun Melsungen AG:
One of the leading companies in the infusion technology segment, B. Braun Melsungen AG is known for its development of innovative and reliable medical devices including MRI compatible IV infusion pumps.Fresenius Kabi AG:
Fresenius Kabi specializes in infusion therapy and clinical nutrition, providing a range of MRI compatible infusion pumps that ensure patient safety and effective drug delivery.Smiths Medical:
A prominent player in the medical devices industry, Smiths Medical is recognized for its advanced infusion systems that comply with MRI safety standards.CareFusion (Becton, Dickinson and Company):
CareFusion develops advanced medical technologies and infusion systems with a focus on accuracy and safety, offering MRI compatible solutions for healthcare providers.Medtronic :
Medtronic focuses on developing breakthrough medical technologies, including MRI compatible infusion pumps that align with evolving healthcare needs.We're grateful to work with incredible clients.









FAQs
What is the market size of mri Compatible Iv Infusion Pumps?
The MRI-compatible IV infusion pumps market is valued at approximately $1.5 billion as of 2023, with a projected CAGR of 7.8% through 2033. This growth reflects increasing demand and technological advancements in medical devices.
What are the key market players or companies in this mri Compatible Iv Infusion Pumps industry?
Key players in the MRI-compatible IV infusion pumps market include leading manufacturers known for innovative medical device solutions. These companies play a crucial role in advancing technologies to ensure safety and efficiency during MRI procedures.
What are the primary factors driving the growth in the mri Compatible Iv Infusion Pumps industry?
Growth in the MRI-compatible IV infusion pumps sector is driven by rising healthcare expenditures, the increasing prevalence of chronic diseases, advancements in MRI technology, and a growing emphasis on patient safety and care quality in medical settings.
Which region is the fastest Growing in the mri Compatible Iv Infusion Pumps?
The North America region is currently the fastest-growing market for MRI-compatible IV infusion pumps, projected to increase from $0.54 billion in 2023 to $1.17 billion by 2033, driven by technological innovations and strong healthcare infrastructure.
Does ConsaInsights provide customized market report data for the mri Compatible Iv Infusion Pumps industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the MRI-compatible IV infusion pumps industry. Clients can access data focusing on particular segments, regions, or market dynamics for strategic insights.
What deliverables can I expect from this mri Compatible Iv Infusion Pumps market research project?
The deliverables from this market research project include comprehensive reports detailing market size, growth analysis, competitive landscape, segment performance, regional insights, and future trends, equipping stakeholders for informed decision-making.
What are the market trends of mri Compatible Iv Infusion Pumps?
Emerging trends in the MRI-compatible IV infusion pumps market include an increased focus on smart infusion technologies, improved integration with health IT systems, enhanced safety features, and a shift towards collaborative care models that prioritize patient outcomes.